Evaluating the Feasibility of Hydrogel-Based Neural Cell Sprays
Abstract
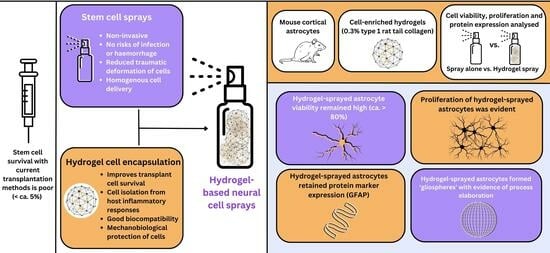
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrogel Spray Preparation
2.3. Derivation of Primary Astrocyte Cultures
2.4. Cell Delivery
2.5. Cell and Hydrogel Biomatrix Co-Visualisation Using Double Staining
2.6. Assessment of Astrocyte Viability
2.7. Assessment of Astrocyte Proliferation
2.8. Assessment of Astrocyte-Specific Protein Marker (GFAP) Expression
2.9. Cell imaging, Quantification and Statistical Analysis
3. Results
3.1. 0.3% Collagen Solutions Showed Optimal Properties for Spray Delivery: Co-Staining Revealed Cell Clusters within the Biomatrix
3.2. Sprayed Intra-Gel Astrocytes Show High Viability
3.3. Sprayed Intra-Gel Astrocytes Proliferate, Retain Marker Expression and Tend to Cluster (Putative “Gliospheres”)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silver, J.; Schwab, M.E.; Popovich, P.G. Central nervous system regenerative failure: Role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb. Perspect. Biol. 2014, 7, a020602. [Google Scholar] [CrossRef] [PubMed]
- Popescu, C.; Anghelescu, A.; Daia, C.; Onose, G. Actual data on epidemiological evolution and prevention endeavours regarding traumatic brain injury. J. Med. Life 2015, 8, 272–277. [Google Scholar] [PubMed]
- Shin, J.C.; Kim, K.N.; Yoo, J.; Kim, I.S.; Yun, S.; Lee, H.; Jung, K.; Hwang, K.; Kim, M.; Lee, I.S.; et al. Clinical Trial of Human Fetal Brain-Derived Neural Stem/Progenitor Cell Transplantation in Patients with Traumatic Cervical Spinal Cord Injury. Neural Plast. 2015, 2015, 630932. [Google Scholar] [CrossRef] [PubMed]
- Kawabori, M.; Tanimori, A.; Kitta, S.; Shichinohe, H.; Houkin, K. Evaluation of Novel Stereotactic Cannula for Stem Cell Transplantation against Central Nervous System Disease. Stem Cells Int. 2020, 2020, 4085617. [Google Scholar] [CrossRef]
- Zhou, Y.; Shao, A.; Xu, W.; Wu, H.; Deng, Y. Advance of Stem Cell Treatment for Traumatic Brain Injury. Front. Cell Neurosci. 2019, 13, 301. [Google Scholar] [CrossRef]
- Genchi, A.; Brambilla, E.; Sangalli, F.; Radaelli, M.; Bacigaluppi, M.; Furlan, R.; Andolfo, A.; Drago, D.; Magagnotti, C.; Scotti, G.M.; et al. Neural stem cell transplantation in patients with progressive multiple sclerosis: An open-label, phase 1 study. Nat. Med. 2023, 29, 75–85. [Google Scholar] [CrossRef]
- Baloh, R.H.; Johnson, J.P.; Avalos, P.; Allred, P.; Svendsen, S.; Gowing, G.; Roxas, K.; Wu, A.; Donahue, B.; Osborne, S.; et al. Transplantation of human neural progenitor cells secreting GDNF into the spinal cord of patients with ALS: A phase 1/2a trial. Nat. Med. 2022, 28, 1813–1822. [Google Scholar] [CrossRef]
- Tejeda, G.; Ciciriello, A.J.; Dumont, C.M. Biomaterial Strategies to Bolster Neural Stem Cell-Mediated Repair of the Central Nervous System. Cells Tissues Organs 2022, 211, 655–669. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.; Reuss, J.L.; Liu, N.; Wu, C.; Li, J.; Xu, S.; Wang, F.; Hazel, T.G.; Cunningham, M.; et al. Stable Intracerebral Transplantation of Neural Stem Cells for the Treatment of Paralysis Due to Ischemic Stroke. Stem Cells Transl. Med. 2019, 8, 999–1007. [Google Scholar] [CrossRef]
- Li, Y.H.; Feng, L.; Zhang, G.X.; Ma, C.G. Intranasal delivery of stem cells as therapy for central nervous system disease. Exp. Mol. Pathol. 2015, 98, 145–151. [Google Scholar] [CrossRef]
- Harting, M.T.; Jimenez, F.; Xue, H.; Fischer, U.M.; Baumgartner, J.; Dash, P.K.; Cox, C.S. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J. Neurosurg. 2009, 110, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S., Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Nakagomi, N.; Nakagomi, T.; Kubo, S.; Nakano-Doi, A.; Saino, O.; Takata, M.; Yoshikawa, H.; Stern, D.M.; Matsuyama, T.; Taguchi, A. Endothelial cells support survival, proliferation, and neuronal differentiation of transplanted adult ischemia-induced neural stem/progenitor cells after cerebral infarction. Stem Cells. 2009, 27, 2185–2195. [Google Scholar] [CrossRef]
- Toda, H.; Takahashi, J.; Iwakami, N.; Kimura, T.; Hoki, S.; Mozumi-Kitamura, K.; Ono, S.; Hashimoto, N. Grafting neural stem cells improved the impaired spatial recognition in ischemic rats. Neurosci. Lett. 2001, 316, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Wahlberg, B.; Ghuman, H.; Liu, J.R.; Modo, M. Ex vivo biomechanical characterization of syringe-needle ejections for intracerebral cell delivery. Sci. Rep. 2018, 8, 9194. [Google Scholar] [CrossRef]
- Lang, H.M.; Schnabel, L.V.; Cassano, J.M.; Fortier, L.A. Effect of needle diameter on the viability of equine bone marrow derived mesenchymal stem cells. Vet. Surg. 2017, 46, 731–737. [Google Scholar] [CrossRef]
- Woods, W.; Evans, D.; Mogas Barcons, A.; Tzerakis, N.; Adams, C.; Maitreyi Chari, D. Stem cell sprays for neurological injuries: A perspective. Emerg. Top. Life Sci. 2021, 5, 519–522. [Google Scholar] [CrossRef]
- Kalladka, D.; Sinden, J.; Pollock, K.; Haig, C.; McLean, J.; Smith, W.; McConnachie, A.; Santosh, C.; Bath, P.M.; Dunn, L.; et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. Lancet 2016, 388, 787–796. [Google Scholar] [CrossRef]
- Shichinohe, H.; Kawabori, M.; Iijima, H.; Teramoto, T.; Abumiya, T.; Nakayama, N.; Kazumata, K.; Terasaka, S.; Arato, T.; Houkin, K. Research on advanced intervention using novel bone marrow stem cell (RAINBOW): A study protocol for a phase I, open-label, uncontrolled, dose-response trial of autologous bone marrow stromal cell transplantation in patients with acute ischemic stroke. BMC Neurol. 2017, 17, 179. [Google Scholar] [CrossRef]
- Woods, W.A.; Chowdhury, F.; Tzerakis, N.; Adams, C.F.; Chari, D.M. Developing a New Strategy for Delivery of Neural Transplant Populations Using Precursor Cell Sprays and Specialized Cell. Adv. NanoBiomed Res. 2021, 1, 2100051. [Google Scholar] [CrossRef]
- Alvarado-Velez, M.; Pai, S.B.; Bellamkonda, R.V. Hydrogels as carriers for stem cell transplantation. IEEE Trans. Biomed. Eng. 2014, 61, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Chan, A.; Morad, L.; Kornblum, H.I.; Fan, G.; Carmichael, S.T. Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabilit. Neural Repair. 2010, 24, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Baldari, S.; Di Rocco, G.; Piccoli, M.; Pozzobon, M.; Muraca, M.; Toietta, G. Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies. Int. J. Mol. Sci. 2017, 18, 2087. [Google Scholar] [CrossRef] [PubMed]
- Farhat, W.; Hasan, A.; Lucia, L.; Becquart, F.; Ayoub, A.; Kobeissy, F. Hydrogels for advanced stem Cell therapies: A biomimetic materials approach for enhancing natural tissue function. IEEE Rev. Biomed. Eng. 2019, 12, 333–351. [Google Scholar] [CrossRef]
- Kornev, V.A.; Grebenik, E.A.; Solovieva, A.B.; Dmitriev, R.I.; Timashev, P.S. Hydrogel-assisted neuroregeneration approaches towards brain injury therapy: A state-of-the-art review. Comput. Struct. Biotechnol. J. 2018, 16, 488–502. [Google Scholar] [CrossRef]
- Hlavac, N.; Kasper, M.; Schmidt, C.E. Progress toward finding the perfect match: Hydrogels for treatment of central nervous system injury. Mater. Today Adv. 2020, 6, 100039. [Google Scholar] [CrossRef]
- Foster, A.A.; Marquardt, L.M.; Heilshorn, S.C. The Diverse Roles of Hydrogel Mechanics in Injectable Stem Cell Transplantation. Curr. Opin. Chem. Eng. 2017, 15, 15–23. [Google Scholar] [CrossRef]
- Lu, J.; Guan, F.; Cui, F.; Sun, X.; Zhao, L.; Wang, Y.; Wang, X. Enhanced angiogenesis by the hyaluronic acid hydrogels immobilized with a VEGF mimetic peptide in a traumatic brain injury model in rats. Regen. Biomater. 2019, 6, 325–334. [Google Scholar] [CrossRef]
- Aguado, B.A.; Mulyasasmita, W.; Su, J.; Lampe, K.J.; Heilshorn, S.C. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng. Part A 2012, 18, 806–815. [Google Scholar] [CrossRef]
- Yang, K.; Han, Q.; Chen, B.; Zheng, Y.; Zhang, K.; Li, Q.; Wang, J. Antimicrobial hydrogels: Promising materials for medical application. Int. J. Nanomed. 2018, 13, 2217–2263. [Google Scholar] [CrossRef]
- Naomi, R.; Ridzuan, P.M.; Bahari, H. Current Insights into Collagen Type I. Polymers 2021, 13, 2642. [Google Scholar] [CrossRef]
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.F.M.R.; Motta, A.; Fauzi, M.B. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz, R.; Díaz, D.D. Self-Healing Collagen-Based Hydrogel for Brain Injury Therapy. Self-Heal. Self-Recover. Hydrogels Adv. Polym. Sci. 2020, 285, 355–378. [Google Scholar] [CrossRef]
- Carretta, A.; Epskamp, M.; Ledermann, L.; Staartjes, V.E.; Neidert, M.C.; Regli, L.; Stienen, M.N. Collagen-bound fibrin sealant (TachoSil®) for dural closure in cranial surgery: Single-centre comparative cohort study and systematic review of the literature. Neurosurg. Rev. 2022, 45, 3779–3788. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.F.; Delaney, A.M.; Carwardine, D.R.; Tickle, J.; Granger, N.; Chari, D.M. Nanoparticle-Based Imaging of Clinical Transplant Populations Encapsulated in Protective Polymer Matrices. Macromol. Biosci. 2019, 19, e1800389. [Google Scholar] [CrossRef] [PubMed]
- Kummrow, A.; Frankowski, M.; Bock, N.; Werner, C.; Dziekan, T.; Neukammer, J. Quantitative assessment of cell viability based on flow cytometry and microscopy. Cytometry A 2013, 83, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Colodner, K.J.; Montana, R.A.; Anthony, D.C.; Folkerth, R.D.; De Girolami, U.; Feany, M.B. Proliferative potential of human astrocytes. J. Neuropathol. Exp. Neurol. 2005, 64, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Latov, N.; Nilaver, G.; Zimmerman, E.A.; Johnson, W.G.; Silverman, A.J.; Defendini, R.; Cote, L. Fibrillary astrocytes proliferate in response to brain injury: A study combining immunoperoxidase technique for glial fibrillary acidic protein and radioautography of tritiated thymidine. Dev. Biol. 1979, 72, 381–384. [Google Scholar] [CrossRef]
- Guizzetti, M.; Kavanagh, T.J.; Costa, L.G. Measurements of astrocyte proliferation. Methods Mol. Biol. 2011, 758, 349–359. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Jensen, J.B.; Parmar, M. Strengths and limitations of the neurosphere culture system. Mol. Neurobiol. 2006, 34, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Choe, G.; Park, J.; Park, H.; Lee, J.Y. Hydrogel Biomaterials for Stem Cell Microencapsulation. Polymers 2018, 10, 997. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.F.; Hong, M.H.; Ho, R.M.; Chung, C.K.; Lin, Y.H.; Chen, C.H.; Sung, H.W. Novel method using a temperature-sensitive polymer (methylcellulose) to thermally gel aqueous alginate as a pH-sensitive hydrogel. Biomacromolecules 2004, 5, 1917–1925. [Google Scholar] [CrossRef]
- Canton, I.; Warren, N.J.; Chahal, A.; Amps, K.; Wood, A.; Weightman, R.; Wang, E.; Moore, H.; Armes, S.P. Mucin-Inspired Thermoresponsive Synthetic Hydrogels Induce Stasis in Human Pluripotent Stem Cells and Human Embryos. ACS Cent. Sci. 2016, 2, 65–74. [Google Scholar] [CrossRef]
- Bibber, B.; Sinha, G.; Lobba, A.R.; Greco, S.J.; Rameshwar, P. A review of stem cell translation and potential confounds by cancer stem cells. Stem Cells Int. 2013, 2013, 241048. [Google Scholar] [CrossRef]
- Kelly, S.; Bliss, T.M.; Shah, A.K.; Sun, G.H.; Ma, M.; Foo, W.C.; Masel, J.; Yenari, M.A.; Weissman, I.L.; Uchida, N.; et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc. Natl. Acad. Sci. USA 2004, 101, 11839–11844. [Google Scholar] [CrossRef]
- Motamed, S.; Del Borgo, M.P.; Zhou, K.; Kulkarni, K.; Crack, P.J.; Merson, T.D.; Aguilar, M.I.; Finkelstein, D.I.; Forsythe, J.S. Migration and Differentiation of Neural Stem Cells Diverted from the Subventricular Zone by an Injectable Self-Assembling β-Peptide Hydrogel. Front. Bioeng. Biotechnol. 2019, 7, 315. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods. 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Schnabel-Lubovsky, M.; Kossover, O.; Melino, S.; Nanni, F.; Talmon, Y.; Seliktar, D. Visualizing cell-laden fibrin-based hydrogels using cryogenic scanning electron microscopy and confocal microscopy. J. Tissue Eng. Regen. Med. 2019, 13, 587–598. [Google Scholar] [CrossRef]
- Jones, C.G. Scanning electron microscopy: Preparation and imaging for SEM. Methods Mol. Biol. 2012, 915, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, M.Y.; Wu, J.; Zhang, H.; Yang, L.; Lun, D.X.; Hu, Y.C.; Liu, B. Picrosirius-Polarization Method for Collagen Fiber Detection in Tendons: A Mini-Review. Orthop. Surg. 2021, 13, 701–707. [Google Scholar] [CrossRef] [PubMed]




| Collagen Concentration | Spray Consistency | Tubing Blockage | Gelation Capacity |
|---|---|---|---|
| 0.1% Hydrogels | Easily sprayable, no increased resistance, consistent sprays. | No blockage of spray tubing observed. | No gelation observed at any time points. |
| 0.3% Hydrogels | Sprayable with minor increased resistance. Consistent sprays. | No blockage of spray tubing observed. | Fibrillary gelation observed after 2 h of incubation at 37 °C |
| 0.6% Hydrogels | Sprayable but with high levels of resistance. Inconsistent low-volume sprays. | Evidence of tubing blockage observed. | Fibrillary gelation observed after 2 h of incubation at 37 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, D.; Barcons, A.M.; Basit, R.H.; Adams, C.; Chari, D.M. Evaluating the Feasibility of Hydrogel-Based Neural Cell Sprays. J. Funct. Biomater. 2023, 14, 527. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb14100527
Evans D, Barcons AM, Basit RH, Adams C, Chari DM. Evaluating the Feasibility of Hydrogel-Based Neural Cell Sprays. Journal of Functional Biomaterials. 2023; 14(10):527. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb14100527
Chicago/Turabian StyleEvans, Daisy, Aina Mogas Barcons, Raja Haseeb Basit, Christopher Adams, and Divya Maitreyi Chari. 2023. "Evaluating the Feasibility of Hydrogel-Based Neural Cell Sprays" Journal of Functional Biomaterials 14, no. 10: 527. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb14100527