1. Introduction
Human bone has multiple functions with hierarchical structural adoptions to perform variable functions [
1]. The two main structures of the bone are the cancellous bone and the outer cortical bone [
2]. The dynamic remodeling process helps heal the bone and adapt to the mechanical loads by absorbing and replacing the old micro-cracked bone. Despite the regenerative properties, bone defects and injuries due to trauma can cause severe diseases (osteoporosis, osteopaenia, and pagets) and nonunion of bone. Dealing with the aging society and maintaining the patient’s quality and activities of life have led to the utilization of orthopedic implants.
Bone fixation and implant materials made from various metals like stainless steel, cobalt, and machined titanium alloy have been extensively used. However, these materials often encounter issues such high elastic modulus as compared to the bone. To address these challenges, there is a growing trend in the use of 3D-printed Ti cages that overcomes the problem of elastic modulus. This is particularly noteworthy for non-biodegradable graft materials [
3].
The Ti alloy’s excellent mechanical properties and safety have rendered it an ideal candidate for orthopedic implants. With the advent of 3D printing technology for titanium alloy, implants can be tailored to meet individual needs. One of the popular techniques for 3D-printed Ti implants is selective laser melting (SLM) [
4,
5]. Additionally, direct metal laser sintering (DMLS) has been recently researched for higher resolution and the use of a low-temperature laser compared to SLM, contributing to the successful manufacturing of Ti implants [
6]. The 3D printing of the Ti-implants has several advantages, such as manufacturing cycle, personalized customization, and cost-effectiveness. Nevertheless, these bone implants face many challenges which need to be addressed. One of the challenges is the bio-inertness of titanium-based alloys [
7]. The 3D implants have large holes that are difficult to fill by the cells, thereby displaying delayed bone regeneration.
The source of gelatin (G) is collagen (the most abundant component of ECM) obtained from the bones and skin of animals by chemical and thermal treatment. Therefore, the chemical structure of gelatin is closely related to its precursor collagen [
8]. Gelatin has a linear structure consisting of glycine-X-Y amino acid repeated units. Gelatin retains the RGD (Arg-Gly-Asp) sequence [
9]. RGD is known to help in cell attachment, repairment, integration, and proliferation. RDG, being considered a biomimetic peptide, can accelerate tissue regeneration and protect cells from apoptosis [
10,
11]. Due to the biochemical resemblance of gelatin to collagen, it is degraded by protease enzymes such as metalloproteases and collagenase, thereby rendering it non-toxic to the body [
12,
13].
Hyaluronic acid (H), distributed throughout the human body, is a significant component of ECM contributing to the hydration and stability of cells. As a natural polysaccharide, it offers high biocompatibility and helps in the proliferation of cells. It is constituted of two units, D-glucuronic acid and N-acetyl glucosamine disaccharide, and due to the presence of hydroxyl groups, it is hydrophilic. It can be rendered as a cross-linked hybrid hydrogel with proteins and covalently attach to metallic surfaces for coating purposes [
14]. Through CD44 surface receptor signaling, it is known to promote cell proliferation and movement [
15]. Depending on the molecular weight of H, the physicochemical, biological, and physical properties also vary. H with molecular weight 20–200 kDa displays embryonic development and healing abilities [
16]. In contrast, 800 kDa to 1900 kDa H helps in osteogenic differentiation and new bone formation [
17,
18].
A combination of G-H has been used to prepare a scaffold for bone regeneration in combination with various biomaterials showing remarkable biological activities. G-H offers a unique physical and biological feature that elevates the innate ability of the body to regenerate cells. Especially they coordinate cell migration, proliferation, and adhesion for bone regeneration [
19,
20]. The ability of G-H to help in cell migration makes them a reliable material to fill the holes in the metal-based scaffold. It acts as a bridging material in assisting cells to penetrate and proliferate through the large holes, which, if left unfilled, may result in late bone formation.
There are many reports on ways to increase the biocompatibility of Ti-based implants by coating, chemical modification, and loading of the bioactive materials [
21,
22,
23]. Although loading bioactive materials helps elevate the titanium-based scaffolds’ biocompatibility, the 3D-printed Ti scaffolds still have holes. For the cell penetration in the 3D-printed Ti scaffold, components that not only increase the scaffold’s biocompatibility but also act as a bridge that helps the cell migrate toward the holes for new bone formation are needed.
In this study, we have developed a 3D-printed Ti cage loaded with G-H. G-H loading can fill the vacant holes of the scaffold, which can increase biocompatibility and cell penetration. We hypothesized that by loading G-H to a 3D-printed Ti cage, the scaffold’s biocompatibility would be increased, and vacant holes of the scaffold would be filled, which would help better cell penetration. It was hypothesized that G-H would act as a bridging component for the cells to proliferate, resulting in early bone formation compared to the empty scaffold with delayed bone regeneration as cells cannot easily migrate to the large vacant holes. We evaluated our hypothesis based on the in vitro biocompatibility tests and in vivo implantation in the rabbit model for one and two months. The results supported our view that the G-H-bridged Ti cage shows higher bone formation than the hollow Ti cage.
2. Materials and Methods
2.1. Materials
Ti-6Al-4V ELI alloy powder (ASTM standard, grade 23) was used to fabricate the 3D- printed samples in this study. Gelatin and hyaluronic acid (mol. wt. ~1.5–1.8 × 106 Da) was acquired from Sigma-Aldrich, St. Louis, MO, USA. MC3T3-E1 cells were obtained from ATCC (pre-osteoblast ATCC, CRL-2593, the American Type Culture Collection, Manassas, VA, USA). α-MEM minimum essential medium and MTT [3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide] solution were acquired from Gibco, New York, NY, USA. PS used was obtained from Bio-Whittaker, Walkersville, MD, USA. 1% penicillin–streptomycin, 10% fetal bovine serum FBS Bovine serum albumin, PBS, fluorescein isothiocyanate (FITC) conjugated phalloidin solution, Hoechst, and Methyl-methacrylate resin were obtained from Sigma Aldrich, St. Louis, MO, USA. DMSO (dimethyl sulfoxide) was obtained from Samchun Chemical, Pyeongtaek, Republic of Korea. Vinculin antibody was acquired from Millipore, Burlington, MA, USA.
2.2. Sample Preparation
A direct metal laser sintering 3D printer (EOSINT M280, EOS, Krailling, Germany) was used to fabricate a 3D-printed Ti alloy (Ti6Al4V) cage. The dimensions of the cage were 6 mm × 5 mm. Gelatin (Sigma Aldrich, Burlington, MA, USA) 10% (w/v) was dissolved in deionized water. Typically, 0.5% (w/v) of hyaluronic acid (mol. wt. ~1.5–1.8 × 106 Da, Sigma Aldrich, Burlington, MA, USA) was added to the gelatin mixture (volume ratio 15:85). To load G and H, a 3D-printed scaffold was permeated with the mixture. The scaffold was then placed in a freezer at −80 °C and freeze-dried for 24 h.
2.3. Surface Morphology
In preparation for scanning electron microscope (SEM) observation, the samples were pre-processed, involving platinum (Pt) coating under vacuum conditions. To analyze the surface morphology, a scanning electron microscope (JSM-7401F, Tokyo, Japan) equipped with an energy-dispersive X-Ray spectroscope (EDS) was used. Pore diameter and surface area were measured by Autosorb iQ Station 2. An X-ray Photoelectron Spectrometer (Thermo Scientific, Waltham, MA, USA) was used to obtain the XPS data. The scanning range was 0–1350 eV with an AlKα radiation source.
2.4. Mechanical Properties
To evaluate the mechanical properties, a universal testing machine (Shimadzu Corporation (UH-F1000kNX, Kyoto, Japan) was used (diameter = 6 mm, height = 5 mm, n = 3). The deformation rate during compression was 0.5 mm/min.
2.5. In Vitro Biocompatibility
The scaffold’s cell proliferation and bio-availability were tested by employing MC3T3-E1 cells. A humidified incubator at 37 °C was used to maintain the cells with 5% CO2. A cell media that contained α-MEM minimum essential medium, 1% penicillin–streptomycin, 10% fetal bovine serum FBS, and PS was used.
Typically, 1 × 104 MC3T3-E1 cells/mL were directly seeded onto each scaffold to evaluate the cell proliferation ability of the cells. Samples were sterilized before use, and a 24-well plate was used. A time frame of 1, 3, and 7 days was employed to compare cell proliferation. For visualization of the cells, cells were washed with PBS thrice at the predetermined time intervals. Fixation was performed with 4% paraformaldehyde for 10 min. For permeabilization, 0.5% Triton X-100 (Sigma Aldrich) was used for 10 min. Bovine serum albumin (BSA Sigma-Aldrich) was used as a blocking agent for 1 hr at ambient temperature. For immunostaining, fluorescein isothiocyanate (FITC) conjugated phalloidin solution was used at a concentration of 25 μg/mL with an incubation time of 12 h at 4 °C. Hoechst (Sigma Aldrich) was used for nuclei staining. A confocal microscope (Olympus, FV10i-W, Center Valley, PA, USA) equipped with FV10i-ASW2.0 software was used to visualize the cells.
For cell viability assay, cell-seeded samples were removed from the incubator at the predetermined time, and 200 μL MTT [3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide] solution (Gibco) was added, followed by incubation at 37 °C for 4 h. After addition of DMSO (dimethyl sulfoxide, Samchun Chemical, Pyeongtaek, Republic of Korea), samples were incubated for 1 hr. An ELISA reader (EL 312, Biokinetics reader, Bio-Tek instrument, Winooski, VT, USA) was used to determine the optical density at 595 nm.
Cell adhesion behavior was evaluated by seeding 1 × 105 cells on the scaffold and incubated for 24 h at 37 °C. Up till BSA blocking, the same protocol was used as in the cell proliferation section. Vinculin antibody (Millipore) was used for immunostaining overnight at 4 °C, followed by phalloidin conjugated FITC (1 h) and Hoechst (5 min) staining. A confocal microscope was used to visualize the samples.
2.6. Cell Migration
The cell migration behavior in vitro was evaluated in the presence of G-H by seeding MC3T3E1 cells at a density of 104 cells per well in a 6-well plate. The control was an empty well; other wells were coated with G-H gel. The confluent monolayer was established by incubating the well plates with cells. A 1 mL pipette tip was used to scratch the monolayer mechanically. PBS was gently used to wash the cell debris thrice. After the scratch formation, the width between the edges of the defect was measured immediately. Cells were then incubated for 24 h, and the distance was measured again. An inverted IX91 Olympus microscope was used to document the migration photographically. Three different points were used to calculate the average gap.
2.7. RNA Expression
The cells (MC3T3-E1) were seeded on the samples using the osteogenic media [
24]. Afterward, RNA was extracted according to the manufacturer’s protocol (ReboEX, Geneall, Seoul, Korea and Hybrid R, Geneall, Seoul, Republic of Korea). To determine the total concentration, the RNA nanodrop (Thermo Fisher Scientific, Waltham, MA, USA) was employed. RNA was converted to cDNA by Maxime RT PreMix kit (LiliF, Seongnam, Republic of Korea). The gene expression was determined using a StepOneTM RealTime PCR System (Thermo Fisher, Waltham, MA, USA) equipped with PowerUp™ SYBR™ Green Master Mix (Applied Biosystems, Woburn, MA, USA). The expression of gene markers OCN (Osteocalcin), ALP (Alkaline phosphatase), COL1 (collagen 1), and Runx2 was determined.
Table 1 shows the sequence of the primers.
2.8. In Vivo Biocompatibility
The in vivo experiment was conducted using New Zealand white rabbits (12 weeks, ~2.5 kg). Before the animal experiment was conducted, approval was obtained from the Soonchunhyang University Institutional Animal Care and Use Committee (Approval number: SCH22-0120, approval date 5 October 2022). Isoflurane (Piramal Critical Care Inc., Bethlehem, PA, USA) was utilized to anesthetize the animals. The right leg of the rabbit was shaved to make an incision. For sterilization of the site, 70% ethanol was used, followed by a povidone–iodine solution. A hole was made using a trephine drill, and the samples were placed at the defect site, followed by suturing. CO2 inhalation was used to sacrifice the animals after 1 and 2 months. The implanted site was harvested. Typically, 10% buffered formalin was used to fix the samples.
2.9. Micro-CT Analysis
Micro-CT was conducted using a SkyScan 1172 (Bruker, Billerica, MA, USA) scanner. The scanned data were constructed using the software NRecon. CTAn (Bruker, Billerica, MA, USA) software was used to investigate bone formation.
2.10. Histological Analysis
Methyl-methacrylate resin (Sigma Aldrich) was used to fix the samples after dehydration. A diamond abrasive cutter was used to cut the samples, followed by polishing at a thickness of 40 μm. Hematoxylin, eosin, and Masson’s Goldner trichrome staining were used. An BX53 (Olympus, Japan, Tokyo), equipped with a DP72 digital camera, was utilized to capture images.
2.11. Statistical Analysis
All the experiments were conducted in triplicate (n = 3 unless mentioned otherwise). All the statistical analyses were performed using GraphPad Prism version 8.0 (GraphPad Software Inc., San Diego, CA, USA). T-test and ANOVA two variance analysis were used to compare different groups. For confidence level, p < 0.05 was considered.
4. Discussion
3D-printed Ti cages have large holes which are difficult for cells to fill, hence delaying the process of bone regeneration. G-H loading resolves this problem by the formation of the network of pores that act as bridges for cells to penetrate and fill the big holes of the 3D-printed Ti cage. For this purpose, G-H was loaded on the Ti 3D-printed cage. Opting for hyaluronic acid, a more cost-effective yet active material to simulate the extracellular matrix (ECM), sets this study apart from others that mainly focus on loading growth factors into gelatin or incorporating materials from the calcium phosphate family for bone regeneration [
25,
26]. The G-H network filled the Ti cage like small bridges, making a highly interconnected web-like appearance as displayed in the SEM image (
Figure 1A). The G-H scaffold not only provides the physical support to the cells to proliferate, but gelatin and hyaluronic acid are abundant in proteins such as RGD, which helps cells in the fast migration. When cells are loaded on the bare Ti scaffold and G-H-loaded Ti scaffold, the cells proliferate on the G-H scaffold, whereas in the case of the Ti scaffold, the margins are occupied. The well-spreading of cells on the G-H-loaded scaffold can be related to the increased biocompatibility of the scaffold, where the interconnected cytoskeletal network developed as the large square holes of the scaffold got interconnected by the bridging web-like network of the G-H. Based on the confocal images (
Figure 3A), it can be said that cells migrated well between the square holes of the G-H-loaded Ti cage due to the bridging provided by the G-H network. Hence the bridging effect supported the fast proliferation of the cells in the inner zone of the Ti cage. Gelatin is sourced from collagen, and hyaluronic acid is a significant component of the ECM. The combined effect of G and H results in enhanced biocompatibility. Cells can proliferate and adhere in the presence of G-H. Gelatin is known to retain the RGD (Arg-Gly-Asp) sequence, which helps cells in proliferation and adherence.
In vitro analyses suggested that G-H not only increased the scaffold’s biocompatibility but also provided a rather unique network of bridges that helped cells proliferate and penetrate the square holes of the cage. In the case of the bare Ti cage, the square holes were large, hindering the cells’ fast proliferation to fill the holes, whereas after loading of G-H, the holes were filled by the network of bridges, which fastened the process of cell proliferation. It can be assumed that this mechanism of fast proliferation of the cells can also help in the fast regeneration of bone in the in vivo model.
In the comparative analysis of RNA expression levels using RT-PCR, a remarkable increase in the expression levels of ALP, OCN, and Col was observed in the G-H scaffold at the 1 week time point. This observation suggests that the G-H-loaded scaffold induces the entry of pre-osteoblasts into the process of osteoblast differentiation) [
27]. Although similar expression levels were observed at 2 weeks in both scaffolds, the significantly increased expression of Runx2 in the G-H-loaded samples at this time implies a rapid differentiation of pre-osteoblasts into immature osteoblasts induced by the loading of G-H [
28].
The micro-CT visualization supports the hypothesis that the G-H-loaded scaffold can help in the fast proliferation of cells, which can facilitate fast bone regeneration. Cell proliferation due to the bridging network created by G-H assisted in filling the square holes of the scaffold vastly compared to the bare Ti scaffold. In the case of the bare Ti cage, the square holes were difficult to fill, resulting in delayed bone formation.
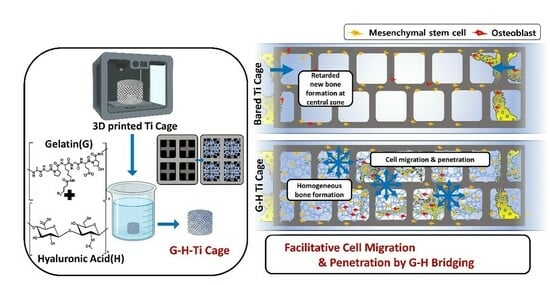
Figure 7 shows the schematic summary of this work. The bare Ti cage is a hollow structure with large-sized pores. Although Ti is a biocompatible material, it did not elevate the innate bone regeneration ability. Only Ti implantation can result in bone formation along the margins of the defect, leaving the inner zone filled with sponge-like marrow. By loading natural G-H gel, a small interconnected network of pores was generated in the Ti cage resulting in the filling of the large pores. This network of pores acts as a bridge that helps the bone cells penetrate the defect’s central zone and results in homogeneous bone formation. The results support the strategy that filling the Ti cage with G-H is an alluring approach for bone regeneration.