Development of Chitosan Functionalized Magnetic Nanoparticles with Bioactive Compounds
Abstract
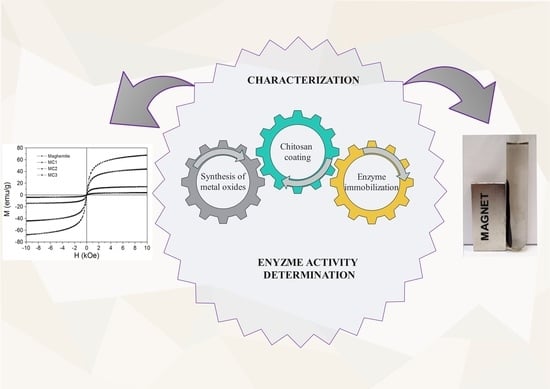
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Magnetic Maghemite Nanoparticles and Magnetic Fluid
2.3. Synthesis of Chitosan Functionalization
2.3.1. Microemulsion Process
2.3.2. Suspension Cross-Linking Process
2.3.3. Covalent Binding Method
2.4. Characterization of Metal Oxide Nanoparticles and Chitosan Functionalized Metal Oxide Micro- and Nanoparticles
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Transmission Electronic Microscopy (TEM) with Energy Dispersive X-ray Analysis (EDS)
2.4.3. Particle Size Analysis
2.4.4. Amino Group Determination
2.5. Preparation of GA- and PEHA-Activated Supports
2.6. Immobilization of HRP and ChOx on Chitosan Functionalized Maghemite Nanoparticles
2.6.1. Protein Assay for Immobilization Efficiency
2.6.2. Enzyme Activity Measurements
3. Results and Discussion
3.1. Characterization of Metal Oxide Nanoparticles and Chitosan Functionalized Metal Oxide Micro- and Nanoparticles
3.1.1. Particle Size Analysis, SEM Analysis and TEM Analysis
3.1.2. Magnetic Properties of Chitosan Functionalized and Bare Metal Oxide
3.1.3. Determination of the Number of Available Amino Groups
3.2. Chitosan Functionalized Metal Oxide Nanoparticles for Enzyme Immobilization
3.2.1. Immobilization of HRP and ChOx Using GA as an Activation Reagent
3.2.2. Immobilization of ChOX Using PEHA as Activation Reagent
3.2.3. Stability of Immobilized ChOX
4. Outcome of the Different Coating Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dhal, J.P. Novel Metal Oxide Nanostructures for Adsorption and Photocatalytic Degradation of Organic Dyes from Aqueous Stream. Ph.D. Thesis, Department of Chemistry National Institute of Technology Rourkela, Odisha, India, 2015. [Google Scholar]
- Patil, K.C. Chemistry of Nanocrystalline Oxide Materials: Combustion Synthesis, Properties and Applications; World Scientific: Danvers, MA, USA, 2008; ISBN 978-981-279-314-0. [Google Scholar]
- Sulman, E.M.; Matveeva, V.G.; Bronstein, L.M. Design of biocatalysts for efficient catalytic processes. Curr. Opin. Chem. Eng. 2019, 26, 1–8. [Google Scholar] [CrossRef]
- Janardhanan, S.K.; Ramasamy, I.; Nair, B.U. Synthesis of iron oxide nanoparticles using chitosan and starch templates. Transit. Met. Chem. 2008, 33, 127–131. [Google Scholar] [CrossRef]
- Sahin, S.; Ozmen, I. Determination of optimum conditions for glucose-6-phosphate dehydrogenase immobilization on chitosan-coated magnetic nanoparticles and its characterization. J. Mol. Catal. B Enzym. 2016, 133, S25–S33. [Google Scholar] [CrossRef]
- Naskar, S.; Sharma, S.; Kuotsu, K. Chitosan-based nanoparticles: An overview of biomedical applications and its preparation. J. Drug Deliv. Sci. Technol. 2018, 49. [Google Scholar] [CrossRef]
- Vaseashta, A.K.; Mihailescu, I.N. Functionalized Nanoscale Materials, Devices and Systems; Springer Science & Business Media: Dordrecht, The Netherlands, 2008; ISBN 978-1-4020-8903-9. [Google Scholar]
- Safdar, R.; Omar, A.A.; Arunagiri, A.; Iyyaswami, R.; Thanabalan, M. Potential of Chitosan and its derivatives for controlled drug release applications—A review. J. Drug Deliv. Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Sonin, D.; Pochkaeva, E.; Zhuravskii, S.; Postnov, V.; Korolev, D.; Vasina, L.; Kostina, D.; Mukhametdinova, D.; Zelinskaya, I.; Skorik, Y.; et al. Biological Safety and Biodistribution of Chitosan Nanoparticles. Nanomaterials 2020, 10, 810. [Google Scholar] [CrossRef] [Green Version]
- Osuna, Y.; Gregorio-Jauregui, K.M.; Gaona-Lozano, J.G.; de la Garza-Rodríguez, I.M.; Ilyna, A.; Barriga-Castro, E.D.; Saade, H.; López, R.G. Chitosan-coated magnetic nanoparticles with low chitosan content prepared in one-step. J. Nano Mater. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Samrot, A.V.; Shobana, N.; Durga Sruthi, P.; Sahithya, C.S. Utilization of chitosan-coated superparamagnetic iron oxide nanoparticles for chromium removal. Appl. Water Sci. 2018, 8, 192. [Google Scholar] [CrossRef] [Green Version]
- Laochai, T.; Mooltongchun, M.; Teepoo, S. Design and Construction of Magnetic Nanoparticles Incorporated with a Chitosan and Poly (vinyl) Alcohol Cryogel and its Application for Immobilization of Horseradish Peroxidase. Energy Procedia 2016, 89, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Gu, T.; Wang, J.; Xia, H.; Wang, S.; Yu, X. Direct Electrochemistry and Electrocatalysis of Horseradish Peroxidase Immobilized in a DNA/Chitosan-Fe3O4 Magnetic Nanoparticle Bio-Complex Film. Materials 2014, 7, 1069–1083. [Google Scholar] [CrossRef] [Green Version]
- Lai, G.-S.; Zhang, H.-L.; Han, D.-Y. Amperometric hydrogen peroxide biosensor based on the immobilization of horseradish peroxidase by carbon-coated iron nanoparticles in combination with chitosan and cross-linking of glutaraldehyde. Microchim. Acta 2009, 165, 159–165. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, J.; Tan, S.; Zhao, D.; Huang, Z.; Mi, Y.; Huang, Z. Amperometric Hydrogen Peroxide Biosensor Based on Horseradish Peroxidase Immobilized on Fe3O4/Chitosan Modified Glassy Carbon Electrode. Electroanalysis 2009, 21, 1514–1520. [Google Scholar] [CrossRef]
- Waifalkar, P.P.; Chougale, A.D.; Kollu, P.; Patil, P.S.; Patil, P.B. Magnetic nanoparticle decorated graphene based electrochemical nanobiosensor for H2O2 sensing using HRP. Colloids Surf. B Biointerfaces 2018, 167, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Goswami, P. Application of chitosan beads immobilized Rhodococcus sp. NCIM 2891 cholesterol oxidase for cholestenone production. Process Biochem. 2014, 49, 2149–2157. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Chen, S.-Y.; Lee, C.-A. Amperometric cholesterol biosensors based on carbon nanotube–chitosan–platinum–cholesterol oxidase nanobiocomposite. Sens. Actuators B Chem. 2008, 135, 96–101. [Google Scholar] [CrossRef]
- Yapar, E.; Kayahan, S.K.; Bozkurt, A.; Toppare, L. Immobilizing cholesterol oxidase in chitosan—Alginic acid network. Carbohydr. Polym. 2009, 76, 430–436. [Google Scholar] [CrossRef]
- Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Chitosan-based (Nano) materials for Novel Biomedical Applications. Molecules 2019, 24, 1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshabib, M.; Onaizi, S.A. A review on phenolic wastewater remediation using homogeneous and heterogeneous enzymatic processes: Current status and potential challenges. Sep. Purif. Technol. 2019, 219, 186–207. [Google Scholar] [CrossRef]
- Yu, B.; Cheng, H.; Zhuang, W.; Zhu, C.; Wu, J.; Niu, H.; Liu, D.; Chen, Y.; Ying, H. Stability and repeatability improvement of horseradish peroxidase by immobilization on amino-functionalized bacterial cellulose. Process Biochem. 2019, 79, 40–48. [Google Scholar] [CrossRef]
- Duarte Baumer, J.; Valério, A.; de Souza, S.M.A.G.U.; Erzinger, G.S.; Furigo, A.; de Souza, A.A.U. Toxicity of enzymatically decolored textile dyes solution by horseradish peroxidase. J. Hazard. Mater. 2018, 360, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Hoang Thi, T.T.; Lee, Y.; Le Thi, P.; Park, K.D. Engineered horseradish peroxidase-catalyzed hydrogels with high tissue adhesiveness for biomedical applications. J. Ind. Eng. Chem. 2019, 78, 34–52. [Google Scholar] [CrossRef]
- Alapati, K.; Handanahal, S.S. Characterization of cholesterol oxidase from a marine Streptomyces sp. and its cytotoxicity. Process Biochem. 2020, 89, 175–185. [Google Scholar] [CrossRef]
- Arya, S.K.; Datta, M.; Malhotra, B.D. Recent advances in cholesterol biosensor. Biosens. Bioelectron. 2008, 23, 1083–1100. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Srivastava, M.; Kalita, P.; Malhotra, B.D. A novel ternary NiFe2O4/CuO/FeO-chitosan nanocomposite as a cholesterol biosensor. Process Biochem. 2012, 47, 2189–2198. [Google Scholar] [CrossRef]
- Silva, R.A.; Carmona-Ribeiro, A.M.; Petri, D.F.S. Enzymatic activity of cholesterol oxidase immobilized onto polymer nanoparticles mediated by Congo red. Colloids Surf. B Biointerfaces 2013, 110, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, A.I.; Lee, K.-P.; Ragupathy, D. Development of a stable cholesterol biosensor based on multi-walled carbon nanotubes–gold nanoparticles composite covered with a layer of chitosan–room-temperature ionic liquid network. Biosens. Bioelectron. 2009, 24, 2211–2217. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaushik, A.; Ansari, A.A.; Tiwari, A.; Malhotra, B.D. Multi-walled carbon nanotubes/sol–gel-derived silica/chitosan nanobiocomposite for total cholesterol sensor. Sens. Actuators B Chem. 2009, 137, 727–735. [Google Scholar] [CrossRef]
- Šulek, F.; Knez, Ž.; Habulin, M. Immobilization of cholesterol oxidase to finely dispersed silica-coated maghemite nanoparticles based magnetic fluid. Appl. Surf. Sci. 2010, 256, 4596–4600. [Google Scholar] [CrossRef]
- Križnik, L.; Vasić, K.; Knez, Ž.; Leitgeb, M. Hyper-activation of ß-galactosidase from Aspergillus oryzae via immobilization onto amino-silane and chitosan magnetic maghemite nanoparticles. J. Clean. Prod. 2018, 179, 225–234. [Google Scholar] [CrossRef]
- Podrepšek, G.H.; Knez, Ž.; Leitgeb, M. Different preparation methods and characterization of magnetic maghemite coated with chitosan. J. Nano Part. Res. 2013, 15, 1751. [Google Scholar] [CrossRef]
- Zhu, H.-Y.; Jiang, R.; Xiao, L.; Li, W. A novel magnetically separable gamma-Fe2O3/crosslinked chitosan adsorbent: Preparation, characterization and adsorption application for removal of hazardous azo dye. J. Hazard. Mater. 2010, 179, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jiang, Y.; Huang, K.; Ding, P.; Chen, J. Preparation and properties of magnetic Fe3O4-chitosan nanoparticles. J. Alloys Compd. 2008, 466, 451–456. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Sahu, S.; Banerjee, I.; Das, M.; Mishra, D.; Maiti, T.; Pramanik, P. Synthesis, characterization, and in vitro biological evaluation of highly stable diversely functionalized superparamagnetic iron oxide nanoparticles. J. Nanopart. Res. 2011, 13. [Google Scholar] [CrossRef]
- Zemljič, L.F.; Tkavc, T.; Vesel, A.; Šauperl, O. Chitosan coatings onto polyethylene terephthalate for the development of potential active packaging material. Appl. Surf. Sci. 2013, 265, 697–703. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Cao, L. Carrier-Bound Immobilized Enzymes: Principles, Application and Design; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 978-3-527-60708-2. [Google Scholar]
- Díaz-Hernández, A.; Gracida-Rodríguez, J.; García-Almendárez, B.; Regalado, C.; Núñez, R.; Amaro Reyes, A. Characterization of Magnetic Nanoparticles Coated with Chitosan: A Potential Approach for Enzyme Immobilization. J. Nanomater. 2018, 2018. [Google Scholar] [CrossRef]
- Shukla, A.; Gundampati, R.K.; Jagannadham, M.V. Immobilization of Euphorbia tirucalli peroxidase onto chitosan-cobalt oxide magnetic nanoparticles and optimization using response surface methodology. Int. J. Biol. Macromol. 2017, 102, 384–395. [Google Scholar] [CrossRef]
- López, R.; Pineda, M.; Hurtado, G.; León, R.; Fernández, S.; Saade, H.; Bueno, D. Chitosan-Coated Magnetic Nanoparticles Prepared in One Step by Reverse Microemulsion Precipitation. Int. J. Mol. Sci. 2013, 14, 19636–19650. [Google Scholar] [CrossRef] [Green Version]
- Reddy, D.H.K.; Lee, S.-M. Application of magnetic chitosan composites for the removal of toxic metal and dyes from aqueous solutions. Adv. Colloid Interface Sci. 2013, 201–202, 68–93. [Google Scholar] [CrossRef]
- Khmara, I.; Strbak, O.; Zavisova, V.; Koneracka, M.; Kubovcikova, M.; Antal, I.; Kavecansky, V.; Lucanska, D.; Dobrota, D.; Kopcansky, P. Chitosan-stabilized iron oxide nanoparticles for magnetic resonance imaging. J. Magn. Magn. Mater. 2019, 474, 319–325. [Google Scholar] [CrossRef]
- Fu, C.-C.; Tran, H.N.; Chen, X.-H.; Juang, R.-S. Preparation of polyaminated Fe3O4@chitosan core-shell magnetic nanoparticles for efficient adsorption of phosphate in aqueous solutions. J. Ind. Eng. Chem. 2020, 83, 235–246. [Google Scholar] [CrossRef]
- Gregorio-Jauregui, K.; Pineda, M.; Rivera-Salinas, J.; Hurtado, G.; Saade, H.; Martinez, J.; Ilyina, A.; Ul, R.; López, R. One-Step Method for Preparation of Magnetic Nanoparticles Coated with Chitosan. J. Nanomater. 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yuan, F.; Pan, J.; Jiao, S.; Jin, L.; Cai, H. A novel method for the determination of the degree of deacetylation of chitosan by coulometric titration. Int. J. Biol. Macromol. 2014, 70, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic Nanoparticles: From Design and Synthesis to Real World Applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.; Paschoalin, V.; Mere Del Aguila, E. Chitosan Nanoparticles: Production, Physicochemical Characteristics and Nutraceutical Applications. Rev. Virtual Quim. 2017, 9, 387. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Unsoy, G.; Yalcin, S.; Khodadust, R.; Gündüz, G.; Gunduz, U. Synthesis optimization and characterization of chitosan-coated iron oxide nanoparticles produced for biomedical applications. J. Nano Part. Res. 2012, 14. [Google Scholar] [CrossRef]
- El-kharrag, R.; Halim, S.S.A.; Amin, A.; Greish, Y.E. Synthesis and characterization of chitosan-coated magnetite nanoparticles using a modified wet method for drug delivery applications. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 73–82. [Google Scholar] [CrossRef]
- Sahu, S.; Shera, S.S.; Banik, R.M. Enhanced Reusability of Horseradish Peroxidase Immobilized onto Graphene Oxide/Magnetic Chitosan Beads for Cost Effective Cholesterol Oxidase Assay. Open Biotechnol. J. 2019, 13. [Google Scholar] [CrossRef] [Green Version]
- Cacicedo, M.L.; Manzo, R.M.; Municoy, S.; Bonazza, H.L.; Islan, G.A.; Desimone, M.; Bellino, M.; Mammarella, E.J.; Castro, G.R. Immobilized Enzymes and Their Applications. In Biomass, Biofuels, Biochemicals: Advances in Enzyme Technology; Singh, R.S., Singhania, R.R., Pandey, A., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 7; pp. 169–200. ISBN 978-0-444-64114-4. [Google Scholar]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 2004, 37, 790–802. [Google Scholar] [CrossRef]
- Secundo, F. Conformational changes of enzymes upon immobilisation. Chem. Soc. Rev. 2013, 42, 6250–6261. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhu, L.; Xu, Y.; Shen, L.; Wang, X.; Ding, Y.; Li, Q.; Deng, D. Hydrogen peroxide biosensor based on horseradish peroxidase immobilized on chitosan-wrapped NiFe2O4 nanoparticles. Microchim. Acta 2011, 174, 55–61. [Google Scholar] [CrossRef]
- Bindhu, L.V.; Abraham, E.T. Immobilization of horseradish peroxidase on chitosan for use in nonaqueous media. J. Appl. Polym. Sci. 2003, 88, 1456–1464. [Google Scholar] [CrossRef]
- Cao, X.; Chen, C.; Yu, H.; Wang, P. Horseradish peroxidase-encapsulated chitosan nanoparticles for enzyme-prodrug cancer therapy. Biotechnol. Lett. 2015, 37, 81–88. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Malki, A.L.; Kumosani, T.A.; El-Shishtawy, R.M. Horseradish peroxidase and chitosan: Activation, immobilization and comparative results. Int. J. Biol. Macromol. 2013, 60, 295–300. [Google Scholar] [CrossRef]
- Amidi, M.; Mastrobattista, E.; Jiskoot, W.; Hennink, W.E. Chitosan-based delivery systems for protein therapeutics and antigens. Adv. Drug Deliv. Rev. 2010, 62, 59–82. [Google Scholar] [CrossRef]
- Labus, K.; Wolanin, K.; Radosiński, Ł. Comparative Study on Enzyme Immobilization Using Natural Hydrogel Matrices—Experimental Studies Supported by Molecular Models Analysis. Catalysts 2020, 10, 489. [Google Scholar] [CrossRef]
- Mateo, C.; Torres, R.; Fernández-Lorente, G.; Ortiz, C.; Fuentes, M.; Hidalgo, A.; López-Gallego, F.; Abian, O.; Palomo, J.M.; Betancor, L.; et al. Epoxy-amino groups: A new tool for improved immobilization of proteins by the epoxy method. Biomacromolecules 2003, 4, 772–777. [Google Scholar] [CrossRef]
- Shinya, S.; Fukamizo, T. Interaction between chitosan and its related enzymes: A review. Int. J. Biol. Macromol. 2017, 104, 1422–1435. [Google Scholar] [CrossRef]
- Faccio, G. From Protein Features to Sensing Surfaces. Sensors 2018, 18, 1204. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Lopez, L.; Pedrero, S.G.; Lopez-Carrobles, N.; Gorines, B.; Virgen-Ortíz, J.; Fernandez-Lafuente, R. Effect of protein load on stability of immobilized enzymes. Enzym. Microb. Technol. 2017, 98, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, H.; Zhang, P.; Zhang, P.; Li, M.; Ding, L. Immobilization of cholesterol oxidase on magnetic fluorescent core-shell-structured nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ahmad, R.; Gautam, V.K.; Khare, S.K. Cholesterol-oxidase-magnetic nanobioconjugates for the production of 4-cholesten-3-one and 4-cholesten-3, 7-dione. Bioresour. Technol. 2018, 254, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Immobilization as a Strategy for Improving Enzyme Properties-Application to Oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef] [Green Version]
- Gracida, J.; Arredondo-Ochoa, T.; García-Almendárez, B.E.; Escamilla-García, M.; Shirai, K.; Regalado, C.; Amaro-Reyes, A. Improved Thermal and Reusability Properties of Xylanase by Genipin Cross-Linking to Magnetic Chitosan Particles. Appl. Biochem. Biotechnol. 2019, 188, 395–409. [Google Scholar] [CrossRef]
- Bharathi, D.; Ranjithkumar, R.; Vasantharaj, S.; Chandarshekar, B.; Bhuvaneshwari, V. Synthesis and characterization of chitosan/iron oxide nanocomposite for biomedical applications. Int. J. Biol. Macromol. 2019, 132, 880–887. [Google Scholar] [CrossRef]
- Maldonado-Camargo, L.; Unni, M.; Rinaldi, C. Magnetic Characterization of Iron Oxide Nanoparticles for Biomedical Applications. Methods Mol. Biol. 2017, 1570, 47–71. [Google Scholar] [CrossRef]
- Kong, X. Simultaneous determination of degree of deacetylation, degree of substitution and distribution fraction of –COONa in carboxymethyl chitosan by potentiometric titration. Carbohydr. Polym. 2012, 88, 336–341. [Google Scholar] [CrossRef]
- Dos Santos, Z.M.; Caroni, A.L.P.F.; Pereira, M.R.; da Silva, D.R.; Fonseca, J.L.C. Determination of deacetylation degree of chitosan: A comparison between conductometric titration and CHN elemental analysis. Carbohydr. Res. 2009, 344, 2591–2595. [Google Scholar] [CrossRef]









| Parameters/Method | Microemulsion Process (MC1) | Suspension Cross-Linking Process (MC2) | Covalent Binding (MC3) |
|---|---|---|---|
| Process Temperature | 40 °C, then 70 °C | room temperature | room temperature |
| Stirring | Ultrasonic (30 min), mechanical (120 min) | Ultrasonic (30 min), mechanical (4 h) | Ultrasonic (60 min), mechanical (12 h) |
| Size distribution | 5–350 μm | 10–200 μm | 50–100 nm |
| Mean diameter | 68.5 μm | 44.2 μm | 58.8 nm |
| Saturation magnetization (Ms) | 4.0 emu/g | 44.1 emu/g | 14.2 emu/g |
| Properties | MC2 | MC3 |
|---|---|---|
| Mean diameter | 44.2 µm | 58.8 nm |
| Saturation magnetization | 44.1 emu/g | 14.2 emu/g |
| Available amino groups | 0.02 mmol/g | 2.48 mmol/g |
| Immobilization efficiency | 35.0% | 37.2% |
| Residual activity | 79.0% | 47.1% |
| Reusability after 7 cycle | 0% | 13% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hojnik Podrepšek, G.; Knez, Ž.; Leitgeb, M. Development of Chitosan Functionalized Magnetic Nanoparticles with Bioactive Compounds. Nanomaterials 2020, 10, 1913. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10101913
Hojnik Podrepšek G, Knez Ž, Leitgeb M. Development of Chitosan Functionalized Magnetic Nanoparticles with Bioactive Compounds. Nanomaterials. 2020; 10(10):1913. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10101913
Chicago/Turabian StyleHojnik Podrepšek, Gordana, Željko Knez, and Maja Leitgeb. 2020. "Development of Chitosan Functionalized Magnetic Nanoparticles with Bioactive Compounds" Nanomaterials 10, no. 10: 1913. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10101913