Characterization and Manipulation of Carbon Precursor Species during Plasma Enhanced Chemical Vapor Deposition of Graphene
Abstract
:1. Introduction
2. Materials and Methods
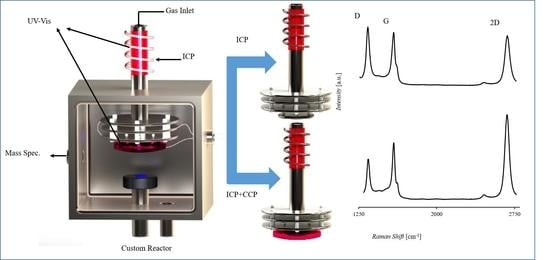
2.1. Reactor Configuration and Capabilities
2.2. Reaction Chamber Characterization
2.3. Catalyst Deposition and Graphene Synthesis
2.4. Graphene Transfer and Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Magnuson, C.W.; Venugopal, A.; Tromp, R.M.; Hannon, J.B.; Vogel, E.M.; Colombo, L.; Ruoff, R.S. Large-Area Graphene Single Crystals Grown by Low-Pressure Chemical Vapor Deposition of Methane on Copper. J. Am. Chem. Soc. 2011, 133, 2816–2819. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Lin, J.; Peng, Z.; Sun, Z.; Zhu, Y.; Li, L.; Xiang, C.; Samuel, E.L.; Kittrell, C.; Tour, J.M. Toward the Synthesis of Wafer-Scale Single-Crystal Graphene on Copper Foils. ACS Nano 2012, 6, 9110–9117. [Google Scholar] [CrossRef] [PubMed]
- Mattevi, C.; Kim, H.; Chhowalla, M. A review of chemical vapour deposition of graphene on copper. J. Mater. Chem. 2011, 21, 3324–3334. [Google Scholar] [CrossRef]
- Shi, B.-Y.; Cao, Q.-J.; Wang, Q.; Han, X.; Wu, H.-F.; Chu, L.-Q.; Fang, Z.-B.; Huang, H.; Tang, J.-X.; Dou, W.-D. Asymmetric Growth of Tetragon-Shaped Single-Crystalline Graphene Flakes on Copper Foil by Annealing Treatment under Oxygen-Free Conditions. J. Phys. Chem. C 2019, 123, 2642–2650. [Google Scholar] [CrossRef]
- Naghdi, S.; Rhee, K.Y.; Park, S.J. A catalytic, catalyst-free, and roll-to-roll production of graphene via chemical vapor deposition: Low temperature growth. Carbon 2018, 127, 1–12. [Google Scholar] [CrossRef]
- Kun, X.; Chen, X.; Jun, D.; Yanxu, Z.; Weiling, G.; Mingming, M.; Lei, Z.; Jie, S. Graphene transparent electrodes grown by rapid chemical vapor deposition with ultrathin indium tin oxide contact layers for GaN light emitting diodes. Appl. Phys. Lett. 2013, 102, 162102. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; He, C.; Zhang, L.; Wang, Y.; Shi, Z.; Cheng, M.; Xie, G.; Wang, D.; Yang, R.; Shi, D.; et al. Growth, Characterization, and Properties of Nanographene. Small 2012, 8, 1429–1435. [Google Scholar] [CrossRef]
- Chan, S.-H.; Chen, S.-H.; Lin, W.-T.; Li, M.-C.; Lin, Y.-C.; Kuo, C.-C. Low-temperature synthesis of graphene on Cu using plasma-assisted thermal chemical vapor deposition. Nanoscale Res. Lett. 2013, 8, 285. [Google Scholar] [CrossRef] [Green Version]
- Zeller, P.; Henß, A.-K.; Weinl, M.; Diehl, L.; Keefer, D.; Lippmann, J.; Schulz, A.; Kraus, J.; Schreck, M.; Wintterlin, J. Detachment of CVD-grown graphene from single crystalline Ni films by a pure gas phase reaction. Surf. Sci. 2016, 653, 143–152. [Google Scholar] [CrossRef]
- Lee, C.-S.; Shin, K.W.; Song, H.-J.; Park, H.; Cho, Y.; Im, D.-H.; Lee, H.; Lee, J.-H.; Won, J.Y.; Chung, J.G.; et al. Fabrication of Metal/Graphene Hybrid Interconnects by Direct Graphene Growth and Their Integration Properties. Adv. Electron. Mater. 2018, 4, 1700624. [Google Scholar] [CrossRef]
- Cui, S.; Cui, C.; Xie, J.; Liu, S.; Shi, J. Carbon fibers coated with graphene reinforced TiAl alloy composite with high strength and toughness. Sci. Rep. 2018, 8, 2364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tracy, J.; Zietz, O.; Olson, S.; Jiao, J. Plasma-Enhanced Chemical Vapor Deposition of Acetylene on Codeposited Bimetal Catalysts Increasing Graphene Sheet Continuity Under Low-Temperature Growth Conditions. Nanoscale Res. Lett. 2019, 14, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, Y.S.; Seo, D.H.; Yeon, D.-H.; Heo, J.; Chung, H.-J.; Benayad, A.; Chung, J.-G.; Han, H.; Lee, H.-S.; Seo, S.; et al. Low temperature growth of complete monolayer graphene films on Ni-doped copper and gold catalysts by a self-limiting surface reaction. Carbon 2013, 64, 315–323. [Google Scholar] [CrossRef]
- Seah, C.-M.; Chai, S.-P.; Mohamed, A.R. Mechanisms of graphene growth by chemical vapour deposition on transition metals. Carbon 2014, 70, 1–21. [Google Scholar] [CrossRef]
- Cabrero-Vilatela, A.; Weatherup, R.S.; Braeuninger-Weimer, P.; Caneva, S.; Hofmann, S. Towards a general growth model for graphene CVD on transition metal catalysts. Nanoscale 2016, 8, 2149–2158. [Google Scholar] [CrossRef] [Green Version]
- Mehedi, H.-A.; Baudrillart, B.; Alloyeau, D.; Mouhoub, O.; Ricolleau, C.; Pham, V.D.; Chacon, C.; Gicquel, A.; Lagoute, J.; Farhat, S. Synthesis of graphene by cobalt-catalyzed decomposition of methane in plasma-enhanced CVD: Optimization of experimental parameters with Taguchi method. J. Appl. Phys. 2016, 120, 065304. [Google Scholar] [CrossRef]
- Lavin-Lopez, M.P.; Fernandez-Diaz, M.; Sanchez-Silva, L.; Valverde, J.L.; Romero, A. Improving the growth of monolayer CVD-graphene over polycrystalline iron sheets. New J. Chem. 2017, 41, 5066–5074. [Google Scholar] [CrossRef]
- Weatherup, R.S.; Dlubak, B.; Hofmann, S. Kinetic Control of Catalytic CVD for High-Quality Graphene at Low Temperatures. ACS Nano 2012, 6, 9996–10003. [Google Scholar] [CrossRef] [Green Version]
- Weatherup, R.S.; Bayer, B.C.; Blume, R.; Ducati, C.; Baehtz, C.; Schlögl, R.; Hofmann, S. In Situ Characterization of Alloy Catalysts for Low-Temperature Graphene Growth. Nano Lett. 2011, 11, 4154–4160. [Google Scholar] [CrossRef]
- Wassei, J.K.; Mecklenburg, M.; Torres, J.A.; Fowler, J.D.; Regan, B.C.; Kaner, R.B.; Weiller, B.H. Chemical Vapor Deposition of Graphene on Copper from Methane, Ethane and Propane: Evidence for Bilayer Selectivity. Small 2012, 8, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lin, L.; Sun, L.; Zhang, J.; Rui, D.; Li, J.; Wang, M.; Tan, C.; Kang, N.; Wei, D.; et al. Low-Temperature and Rapid Growth of Large Single-Crystalline Graphene with Ethane. Small 2018, 14, 1702916. [Google Scholar] [CrossRef]
- Guermoune, A.; Chari, T.; Popescu, F.; Sabri, S.S.; Guillemette, J.; Skulason, H.S.; Szkopek, T.; Siaj, M. Chemical vapor deposition synthesis of graphene on copper with methanol, ethanol, and propanol precursors. Carbon 2011, 49, 4204–4210. [Google Scholar] [CrossRef]
- Li, Z.; Wu, P.; Wang, C.; Fan, X.; Zhang, W.; Zhai, X.; Zeng, C.; Li, Z.; Yang, J.; Hou, J. Low-Temperature Growth of Graphene by Chemical Vapor Deposition Using Solid and Liquid Carbon Sources. ACS Nano 2011, 5, 3385–3390. [Google Scholar] [CrossRef]
- Zhang, B.; Lee, W.H.; Piner, R.; Kholmanov, I.; Wu, Y.; Li, H.; Ji, H.; Ruoff, R.S. Low-Temperature Chemical Vapor Deposition Growth of Graphene from Toluene on Electropolished Copper Foils. ACS Nano 2012, 6, 2471–2476. [Google Scholar] [CrossRef]
- Jang, J.; Son, M.; Chung, S.; Kim, K.; Cho, C.; Lee, B.H.; Ham, M.-H. Low-temperature-grown continuous graphene films from benzene by chemical vapor deposition at ambient pressure. Sci. Rep. 2015, 5, 17955. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, F.; Ruiz, M.; Valderrama, E.; Favre, M.; Bhuyan, H.; Wynham, E.S.; Chuaqui, H. Spectroscopic Characterization Of RF Hydrocarbon Plasmas For DLC Coatings. J. Phys. Conf. Ser. 2014, 511, 012017. [Google Scholar] [CrossRef] [Green Version]
- Denysenko, I.B.; Xu, S.; Long, J.D.; Rutkevych, P.P.; Azarenkov, N.A.; Ostrikov, K. Inductively coupled Ar/CH4/H2 plasmas for low-temperature deposition of ordered carbon nanostructures. J. Appl. Phys. 2004, 95, 2713–2724. [Google Scholar] [CrossRef] [Green Version]
- Baby, A.; Mahony, C.M.O.; Maguire, P.D. Acetylene–argon plasmas measured at a biased substrate electrode for diamond-like carbon deposition: I. Mass spectrometry. Plasma Sources Sci. Technol. 2011, 20, 015003. [Google Scholar] [CrossRef] [Green Version]
- Coyne, B.; Zietz, O.; Olson, S.; Jiao, J. Graphene Layer Control Enabled by Nickel-Copper Alloy Thin Film Catalyst. Microsc. Microanal. 2018, 24, 1624–1625. [Google Scholar] [CrossRef] [Green Version]
- Olson, S.; Zietz, O.; Tracy, J.; Li, Y.; Tao, C.; Jiao, J. Low-temperature chemical vapor deposition growth of graphene films enabled by ultrathin alloy catalysts. J. Vac. Sci. Technol. B 2020, 38, 032202. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Verzhbitskiy, I.; Davey, R.; Casiraghi, C. Raman study on defective graphene: Effect of the excitation energy, type, and amount of defects. Phys. Rev. B 2013, 88, 035426. [Google Scholar] [CrossRef] [Green Version]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the Nature of Defects in Graphene by Raman Spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zietz, O.; Olson, S.; Coyne, B.; Liu, Y.; Jiao, J. Characterization and Manipulation of Carbon Precursor Species during Plasma Enhanced Chemical Vapor Deposition of Graphene. Nanomaterials 2020, 10, 2235. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10112235
Zietz O, Olson S, Coyne B, Liu Y, Jiao J. Characterization and Manipulation of Carbon Precursor Species during Plasma Enhanced Chemical Vapor Deposition of Graphene. Nanomaterials. 2020; 10(11):2235. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10112235
Chicago/Turabian StyleZietz, Otto, Samuel Olson, Brendan Coyne, Yilian Liu, and Jun Jiao. 2020. "Characterization and Manipulation of Carbon Precursor Species during Plasma Enhanced Chemical Vapor Deposition of Graphene" Nanomaterials 10, no. 11: 2235. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10112235