Sterically Hindered Phosphonium Salts: Structure, Properties and Palladium Nanoparticle Stabilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumental
2.2. Materials
2.2.1. Typical Procedure for PdNPs Preparation
2.2.2. Typical Procedure for Suzuki Cross-Coupling
3. Results and Discussion
3.1. Synthetic Procedures
3.2. NMR Spectroscopy
3.2.1. 31P NMR Spectra
3.2.2. 1H NMR Signals for C(α)-H Protons
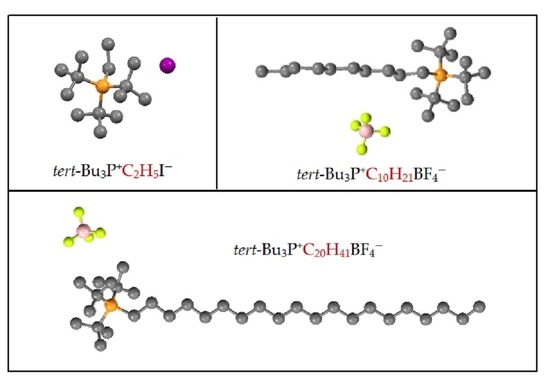
3.3. X-ray Analysis
3.4. Melting Point
3.5. PdNP Stabilization and TEM Sample Preparation
3.6. Catalytic Suzuki Cross-Coupling Reaction
3.7. PdNP Size Dynamics during the Cross-Coupling Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deetlefs, M.; Fanselow, M.; Seddon, K.R. Ionic liquids: The view from Mount Improbable. RSC Adv. 2016, 6, 4280–4288. [Google Scholar] [CrossRef]
- Plechkova, N.; Seddon, K. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Łuczak, J.; Paszkiewicz, M.; Krukowska, A.; Malankowska, A.; Zaleska-Medynska, A. Ionic liquids for nano- and microstructures preparation. Part 1: Properties and multifunctional role. Adv. Colloid Interface Sci. 2016, 230, 13–28. [Google Scholar] [CrossRef]
- Khazalpour, S.; Yarie, M.; Kianpour, E.; Amani, A.; Asadabadi, S.; Seyf, J.Y.; Rezaeivala, M.; Azizian, S.; Zolfigol, M.A. Applications of phosphonium-based ionic liquids in chemical processes. J. Iran Chem. Soc. 2020, 17, 1775–1917. [Google Scholar] [CrossRef]
- Egorova, K.S.; Ananikov, V.P. Fundamental importance of ionic interactions in the liquid phase: A review of recent studies of ionic liquids in biomedical and pharmaceutical applications. J. Mol. Liq. 2018, 272, 271–300. [Google Scholar] [CrossRef]
- Dupont, J. From Molten Salts to Ionic Liquids: A “Nano” Journey. Acc. Chem. Res. 2011, 44, 1223–1231. [Google Scholar] [CrossRef]
- Kashin, A.S.; Galkin, K.I.; Khokhlova, E.A.; Ananikov, V.P. Direct Observation of Self-Organized Water-Containing Structures in the Liquid Phase and Their Influence on 5-(Hydroxymethyl)furfural Formation in Ionic Liquids. Angew. Chem., Int. Ed. 2016, 55, 2161–2166. [Google Scholar] [CrossRef]
- Egorova, K.S.; Posvyatenko, A.V.; Fakhrutdinov, A.N.; Kashin, A.S.; Ananikov, V.P. Assessing possible influence of structuring effects in solution on cytotoxicity of ionic liquid systems. J. Mol. Liq. 2020, 297, 111751. [Google Scholar] [CrossRef]
- Hundertmark, T.; Littke, A.; Buchwald, S.; Fu, G.C. Pd(PhCN)2Cl2/P(t-Bu)3: A Versatile Catalyst for Sonogashira Reactions of Aryl Bromides at Room Temperature. Org. Lett. 2000, 2, 1729–1731. [Google Scholar] [CrossRef]
- Stojanovic, A.; Morgenbesser, C.; Kogelnig, D.; Krachler, R.; Keppler, B.K. Quaternary Ammonium and Phosphonium Ionic Liquids in Chemical and Environmental Engineering. In Ionic Liquids: Theory, Properties, New Approaches; Kokorin, A., Ed.; InTech: London, UK, 2011. [Google Scholar]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Seitkalieva, M.M.; Posvyatenko, A.V.; Khrustalev, V.N.; Ananikov, V.P. Cytotoxic Activity of Salicylic Acid-Containing Drug Models with Ionic and Covalent Binding. ACS Med. Chem. Lett. 2015, 6, 1099–1104. [Google Scholar] [CrossRef]
- Ferraz, R.; Costa-Rodrigues, J.; Fernandes, M.H.; Santos, M.M.; Marrucho, I.M.; Rebelo, L.P.N.; PrudÞncio, C.; Noronha, P.; Petrovski, Z.; Branco, L.C. Antitumor Activity of Ionic Liquids Based on Ampicillin. ChemMedChem 2015, 10, 1480–1483. [Google Scholar] [CrossRef] [Green Version]
- Shamshina, J.L.; Rogers, R.D. Are Myths and Preconceptions Preventing Us from Applying Ionic Liquid Forms of Antiviral Medicines to the Current Health Crisis? Int. J. Mol. Sci. 2020, 21, 6002. [Google Scholar] [CrossRef]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somers, A.E.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. A Review of Ionic Liquid Lubricants. Lubricants 2013, 1, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Osada, I.; de Vries, H.; Scrosati, B.; Passerini, S. Ionic-Liquid-Based Polymer Electrolytes for Battery Applications. Angew.Chem. Int. Ed. 2016, 55, 500–513. [Google Scholar] [CrossRef]
- Khrizanforov, M.; Shekurov, R.; Miluykov, V.; Gilmanova, L.; Kataeva, O.; Yamaleeva, Z.; Gerasimova, T.; Ermolaev, V.; Gubaidullin, A.; Laskin, A.; et al. Excellent supercapacitor and sensor performance of robust cobalt phosphinate ferrocenyl organic framework materials achieved by intrinsic redox and structure properties. Dalt. Trans. 2019, 48, 16986–16992. [Google Scholar] [CrossRef]
- Opallo, M.; Lesniewski, A. A review on electrodes modified with ionic liquids. J. Electroanal. Chem. 2011, 656, 2–16. [Google Scholar] [CrossRef]
- Wassershied, P.; Keim, W. Ionic Liquids-New “Solutions” for Transition Metal Catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- He, Z.; Alexandridis, P. Ionic liquid and nanoparticle hybrid systems: Emerging applications. Adv. Colloid Interface Sci. 2017, 244, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Pensado, A.S.; Pádua, A.A.H. Solvation and Stabilization of Metallic Nanoparticles in Ionic Liquids. Angew. Chem. Int. Ed. 2011, 50, 8683–8687. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.X.; Liebscher, J. Carbon-Carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar] [CrossRef] [PubMed]
- Scholten, J.D.; Caroline Leal, B.; Dupont, J. Transition Metal Nanoparticle Catalysis in Ionic Liquids. ACS Catal. 2012, 2, 184–200. [Google Scholar] [CrossRef]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as Recyclable Catalysts: The Frontier between Homogeneous and Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef]
- Van Vaerenbergh, B.; Lauwaert, J.; Vermeir, P.; Thybaut, J.W.; De Clercq, J. Towards high-performance heterogeneous palladium nanoparticle catalysts for sustainable liquid-phase reactions. React. Chem. Eng. 2020, 5, 1556–1618. [Google Scholar] [CrossRef]
- Hong, K.; Sajjadi, M.; Suh, J.M.; Zhang, K.; Nasrollahzadeh, M.; Jang, H.W.; Varma, R.S.; Shokouhimehr, M. Palladium Nanoparticles on Assorted Nanostructured Supports: Applications for Suzuki, Heck, and Sonogashira Cross-Coupling Reactions. ACS Appl. Nano Mater. 2020, 3, 2070–2103. [Google Scholar] [CrossRef]
- van Deelen, T.; Mejia, C.; de Jong, K. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Kamari, Y.; Ghiaci, M. Incorporation of TiO2 coating on a palladium heterogeneous nanocatalyst. A new method to improve reusability of a catalyst. Cat. Comm. 2016, 84, 16–20. [Google Scholar] [CrossRef]
- Kilic, A.; Gezer, E.; Durap, F.; Aydemir, M.; Baysal, A. Pd(II) supported dioxime functionalized Fe3O4 nanoparticles as efficient, eco-friendly and reusable catalysts for the Suzuki-Miyaura cross-coupling reaction in water. J. Organomet. Chem. 2019, 896, 129–138. [Google Scholar] [CrossRef]
- Abdo, S.F.; Wilson, J.S. Zeolites in Industrial Catalysis. In Zeolites in Catalysis Properties and Applications; Čejka, R.E.M.J., Nachtigall, P., Eds.; RSC: Cambridge, UK, 2017; p. 350. [Google Scholar]
- Azad, M.; Rostamizadeh, S.; Estiri, H.; Nouri, F. Ultra-small and highly dispersed Pd nanoparticles inside the pores of ZIF-8: Sustainable approach to waste-minimized Mizoroki-Heck cross-coupling reaction based on reusable heterogeneous catalyst. App. Organomet. Chem. 2019, 33, 4952. [Google Scholar] [CrossRef]
- Nikoorazm, M.; Khanmoradi, M.; Abdi, Z. A highly efficient palladium complex supported on MCM-41 nanocatalyst for Mizoroki-Heck and Suzuki-Miyaura cross-coupling reaction. J. Iran. Chem. Soc. 2020, 17, 2577–2585. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhu, Z.; Dong, S. Carbon-Based Nanostructures for Advanced Catalysis. ChemCatChem 2015, 7, 2806–2815. [Google Scholar] [CrossRef]
- Kuniyil, M.; Kumar, J.V.S.; Adil, S.F.; Shaik, M.R.; Khan, M.; Assal, M.E.; Siddiqui, M.R.H.; Al-Warthan, A. One-pot synthesized Pd@N-doped graphene: An efficient catalyst for Suzuki-Miyaura couplings. Catalysts 2019, 9, 469. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Dong, W.-H.; Shang, N.-Z.; Feng, C.; Gao, S.-T.; Wang, C. Doped porous carbon supported palladium nanoparticles as a highly efficient and recyclable catalyst for the Suzuki coupling reaction. Chin. Chem. Lett. 2016, 27, 149–154. [Google Scholar] [CrossRef]
- Gogoi, R.; Saikia, R.; Borah, G. Agro waste derived nanosilica supported Pd(ll) complex: A protocol for copper free Sonogashira reaction in water. J. Organomet. Chem. 2019, 897, 80–88. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal–Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2019, 120, 1438–1511. [Google Scholar] [CrossRef]
- Heidari, B.; Heravi, M.M.; Nabid, M.R.; Sedghi, R. Well-dispersed N-heterocyclic carbene-palladium complex anchored onto poly(acrylic acid)/poly(vinyl alcohol) nanofibers: Novel, superior and ecofriendly nanocatalyst for the Suzuki-Miyaura cross-coupling reaction. Appl. Organomet. Chem. 2019, 33, e4934. [Google Scholar] [CrossRef]
- Ghazali-Esfahani, S.; Paunescu, E.; Bagherzadeh, M.; Fei, Z.; Laurenczy, G.; Dyson, P.J. A simple catalyst for aqueous phase Suzuki reactions based on palladium nanoparticles immobilized on an ionic polymer. Sci. China Chem. 2016, 59, 482–486. [Google Scholar] [CrossRef]
- Kandathil, V.; Kempasiddaiah, M.; Sasidhar, B.S.; Patil, S.A. From agriculture residue to catalyst support; A green and sustainable cellulose-based dip catalyst for C–C coupling and direct arylation. Carbohydr. Polym. 2019, 223, 115060. [Google Scholar] [CrossRef]
- Fischer, D.K.; de Fraga, K.R.; Scheeren, C.W. Chitosan microspheres from shrimp waste supporting Pd nanoparticles in ionic liquids: An efficient and eco-friendly catalyst for hydrogenation reactions. J. Nanosci. Nanotechnol. 2020, 20, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, R.; Heidari, B.; Shahmohamadi, H.; Zarshenas, P.; Varma, R.S. Pd nanocatalyst adorned on magnetic chitosan@N-heterocyclic carbene: Eco-compatible Suzuki cross-coupling reaction. Molecules 2019, 24, 3048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi, A.; Sedaghat, T.; Azadi, R.; Motamedi, H. Magnetic Mesoporous Silica Nanocomposite Functionalized with Palladium Schiff Base Complex: Synthesis, Characterization, Catalytic Efficacy in the Suzuki-Miyaura Reaction and α-Amylase Immobilization. Catal. Lett. 2020, 150, 112–126. [Google Scholar] [CrossRef]
- Mahmoudzadeh, M.; Mehdipour, E.; Eisavi, R. MgFe2O4@SiO2-PrNH2/Pd/bimenthonoxime Core-shell Magnetic Nanoparticles as a Recyclable Green Catalyst for Heterogeneous Suzuki Cross-Coupling in Aqueous Ethanol. J. Coord. Chem. 2019, 72, 841–859. [Google Scholar] [CrossRef]
- Zhang, W.; Veisi, H.; Hemmati, S.; Sharifi, R.; Salamat, D.; Karmakar, B.; Hekmati, M.; Zangeneh, M.M.; Zhang, Z.; Su, Q. Fabrication of Pd NPs on pectin-modified Fe3O4 NPs: A magnetically retrievable nanocatalyst for efficient C-C and C-N cross coupling reactions and an investigation of its cardiovascular protective effects. Int. J. Biolog. Macromol. 2020, 160, 1252–1262. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, N. Towards Rational Design of Nanoparticle Catalysis in Ionic Liquids. Catalysts 2013, 3, 543–562. [Google Scholar] [CrossRef] [Green Version]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liquids 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Hoffmann, H.; Schellenbeck, P. Notiz über die Darstellung von Tri-tert.-butylphosphin. Chem. Ber. 1967, 100, 692–693. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Blaschke, G.; Köhler, F.H. Tri(tert-butyl)methylenphosphoran: Konsequenzen sterischer Hinderung für innermolekulare Beweglichkeit und thermischen Zerfallsmechanismus. Z. Naturforsch. 1977, 33b, 757–761. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Blaschke, G.; Zimmer-Gasser, B.; Schubert, U. Extreme sterische Hinderung: Synthese und Struktur des Tetra(tert-butyl)phosphonium-Kations-ein Fall von T-Symmetrie. Chem. Ber. 1980, 113, 1612–1622. [Google Scholar] [CrossRef]
- Goel, R.G.; Ogini, W.O.; Srivastava, R.C. A convenient synthesis of bis(tri-t-butylphosphine)platinum(0) and its oxidative addition and ligand exchange reactions. J. Organomet. Chem. 1981, 214, 405–417. [Google Scholar] [CrossRef]
- Bellinger, G.; Friedrich, H.; Moss, J. Haloalkyl complexes of the transition metals. VI. A study of the reactions of halomethyldicarbonylcyclopentadienyliron complexes with some tertiary phosphine, amine and sulfur ligands. J. Organomet. Chem. 1989, 366, 175–186. [Google Scholar] [CrossRef]
- Das, P.; McNulty, J. Synthetic Approaches to Anti–Inflammatory Macrolactones of the Oxacyclododecindione Type. Eur. J. Org. Chem. 2010, 3587–3591. [Google Scholar] [CrossRef]
- Ermolaev, V.; Miluykov, V.; Rizvanov, I.; Krivolapov, D.; Zvereva, E.; Katsyuba, S.; Sinyashin, O.; Schmutzler, R. Phosphonium ionic liquids based on bulky phosphines: Synthesis, structure and properties. Dalton Trans. 2010, 39, 5564–5571. [Google Scholar] [CrossRef]
- Xu, B.-H.; Yanez, R.; Nakatsuka, H.; Kitamura, M.; Frçhlich, R.; Kehr, G.; Erker, G. Reaction of Frustrated Lewis Pairs with Ketones and Esters. Chem. Asian J. 2012, 7, 1347–1356. [Google Scholar] [CrossRef]
- Adamova, G.; Gardas, R.L.; Rebelo, L.; Robertson, A.J.; Seddon, K.R. Alkyltrioctylphosphonium chloride ionic liquids: Synthesis and physicochemical properties. Dalton Trans. 2011, 40, 12750–12764. [Google Scholar] [CrossRef]
- Adamova, G.; Gardas, R.L.; Nieuwenhuyzen, M.; Puga, A.V.; Rebelo, L.; Robertson, A.J.; Seddon, K.R. Alkyltributylphosphonium chloride ionic liquids: Synthesis, physicochemical properties and crystal structure. Dalton Trans. 2012, 41, 8316–8333. [Google Scholar] [CrossRef]
- Ermolaev, V.; Arkhipova, D.; Nigmatullina, L.; Rizvanov, I.; Milyukov, V.; Sinyashin, O. Palladium Nanoparticles Stabilized by Sterically Hindered Phosphonium Salts as Suzuki Cross-Coupling Catalysts. Russ. Chem. Bull., Int. Ed. 2013, 62, 657–660. [Google Scholar] [CrossRef]
- Khrizanforov, M.; Arkhipova, D.; Shekurov, R.; Gerasimova, T.; Ermolaev, V.; Islamov, D.; Miluykov, V.; Kataeva, O.; Khrizanforova, V.; Sinyashin, O.; et al. Novel paste electrodes based on phosphonium salt room temperature ionic liquids for studying the redox properties of insoluble compounds. J. Solid State Electrochem. 2015, 19, 2883–2890. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT: Integrating space group determination and structure solution. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. 2007, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Van De Streek, J. Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Hartley, F.R. The Chemistry of Organophosphorus Compounds. Volume 3 Phosphonium Salts, Ylides and Phosphoranes; John Wiley & Sons Ltd.: Chichester, UK, 1994; 442p. [Google Scholar]
- Armarego, W.; Chai, C. Purification of Laboratory Chemicals; Butterworth-Heinemann Elsevier Ltd.: Oxford, UK, 2003; p. 609. [Google Scholar]
- Allman, T.; Goel, R. The basicity of phosphines. Can. J. Chem. 1982, 716–722. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Rogers, R.D.; Mantz, R.A.; Trulove, P.C.; Cocalia, V.A.; Visser, A.E.; Anderson, J.L.; Anthony, J.L.; Brennecke, J.F.; Maginn, E.J.; et al. Physicochemical Properties. In Ionic Liquids in Synthesis, 2nd ed.; Wasserscheid, P., Welton, T., Eds.; Wiley-VCH: Weinhein, Germany, 2008; pp. 57–174. [Google Scholar]
- Luska, K.L.; Moores, A. Rational size control of gold nanoparticles employing an organometallic precursor [Au-C≡C-t-Bu]4 and tunable thiolate-functionalized ionic liquids in organic medium. Can. J. Chem. 2012, 90, 145–152. [Google Scholar] [CrossRef]
- Gutel, T.; Santini, C.C.; Philippot, K.; Padua, A.; Pelzer, K.; Chaudret, B.; Chauvin, Y.; Basset, J.M. Organized 3D-alkyl imidazolium ionic liquids could be used to control the size of in situ generated rutheniumnanoparticles? J. Mater. Chem. 2009, 19, 3624–3631. [Google Scholar] [CrossRef]
- Redel, E.; Thomann, R.; Janiak, C. Use of ionic liquids (ILs) for the IL-anion size-dependent formation of Cr, Mo and W nanoparticles from metal carbonyl M(CO)6 precursors. Chem. Commun. 2008, 15, 1789–1791. [Google Scholar] [CrossRef]
- Luska, K.L.; Moores, A. Ruthenium nanoparticle catalysts stabilized in phosphonium and imidazolium ionic liquids: Dependence of catalyst stability and activity on the ionicity of the ionic liquid. Green Chem. 2012, 14, 1736–1742. [Google Scholar] [CrossRef]
- Arkhipova, D.; Ermolaev, V.; Miluykov, V.; Gaynanova, G.; Zakharova, L.; Wagner, G.; Oeckler, O.; Hey-Hawkins, E. Effect of phosphonium ionic liquid/Pd ratio on the catalytic activity of palladium nanoparticles in Suzuki cross-coupling reaction. J. Organometallic. Chem. 2020, 923, 121454. [Google Scholar] [CrossRef]
- Galushko, A.; Gordeev, E.; Kashin, A.S.; Zubavichus, Y.V.; Ananikov, V. Visualization of catalyst dynamics and development of a practical procedure to study complex “cocktail”-type catalytic systems. Faraday Discuss. 2020, accepted. [Google Scholar] [CrossRef]
- Stoyanov, E.S. IR Study of the Structure of Palladium(II) Acetate in Chloroform, Acetic Acid, and their Mixtures in Solution and in Liquid-Solid Subsurface Layers. J. Struct. Chem. 2000, 41, 440–445. [Google Scholar] [CrossRef]
- Hussein, H.E.M.; Ray, A.D.; Macpherson, J.V. Switching on palladium catalyst electrochemical removal from a palladium acetate-acetonitrile system via trace water addition. Green Chem. 2019, 21, 4662–4672. [Google Scholar] [CrossRef]
- Ibragimova, A.; Arkhipova, D.; Vagapova, G.; Ermolaev, V.; Galkina, I.; Nigmatullina, L.; Rizvanov, I.; Zakharova, L.; Milyukov, V.; Konovalov, A.; et al. Influence of the medium self-organization on the catalytic activity of palladium nanoparticles stabilized by amphiphilic phosphonium salts in the Suzuki reaction. Russ. Chem. Bull. Int. Ed. 2014, 63, 1297–1300. [Google Scholar] [CrossRef]
- Ermolaev, V.; Miluykov, V.; Arkhipova, D.; Zvereva, E.; Sinyashin, O. Decyl(Tri-Tert-Butyl)Phosphonium Tetrafluoroborate/Palladium Acetate: An Effective Catalyst for Cross-Coupling Reaction of Arylbromides with Phenylacetylene in Copper-Free Conditions. Phosphorus Sulfur Silicon Relat. Elem. 2013, 188, 168–170. [Google Scholar] [CrossRef]
- Nutzenadel, C.; Zuttel, A.; Chartouni, D.; Schmid, G.; Schlapbach, L. Critical size and surface effect of the hydrogen interaction of palladium clusters. Eur. Phys. J. D 2000, 8, 245–250. [Google Scholar] [CrossRef]
- Eremin, D.B.; Ananikov, V.P. Understanding active species in catalytic transformations: From molecular catalysis to nanoparticles, leaching, “Cocktails” of catalysts and dynamic systems. Coord. Chem. Rev. 2017, 346, 2–19. [Google Scholar] [CrossRef]
- Gnad, C.; Abram, A.; Urstoeger, A.; Weigl, F.; Schuster, M.; Köhler, K. Leaching mechanism of different palladium surface species in Heck reactions of aryl bromides and chlorides. ACS Catal. 2020, 10, 6030–6041. [Google Scholar] [CrossRef]
- Kashin, A.N.; Ganina, O.G.; Cheprakov, A.V.; Beletskaya, I.P. The Direct Non-Perturbing Leaching Test in the Phosphine-Free Suzuki–Miyaura Reaction Catalyzed by Palladium Nanoparticles. ChemCatChem 2015, 7, 2113–2121. [Google Scholar] [CrossRef]
- Schlogl, R. Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2015, 54, 3465–3520. [Google Scholar] [CrossRef] [Green Version]
- Kashin, A.S.; Degtyareva, E.S.; Eremin, D.B.; Ananikov, V.P. Exploring the performance of nanostructured reagents with organic-group-defined morphology in cross-coupling reaction. Nat. Commun. 2018, 9, 2936. [Google Scholar] [CrossRef]
- Azov, V.; Egorova, K.; Seitkalieva, M.; Kashin, A.; Ananikov, V. “Solvent-in-salt” systems for design of new materials in chemistry, biology and energy research. Chem. Soc. Rev. 2018, 47, 1250–1284. [Google Scholar] [CrossRef]

















| Phosphonium Salt | Chemical Shift δ of α-Protons 1H NMR, ppm | Chemical Shift δ 31P NMR, ppm | ||
|---|---|---|---|---|
| N | Cation | Anion | ||
| 1a | t-Bu3P+C2H5 | I− | 2.93 | 50.53 |
| 1b | BF4− | 2.58 | 49.96 | |
| 2a | t-Bu3P+C4H9 | Br− | 2.65 | 49.54 |
| 2b | BF4− | 2.31 | 49.17 | |
| 3a | t-Bu3P+C6H13 | Br− | 2.61 | 49.51 |
| 3b | BF4− | 2.32 | 49.16 | |
| 4a | t-Bu3P+C8H17 | Br− | 2.55 | 49.30 |
| 4b | BF4− | 2.29 | 49.08 | |
| 5a | t-Bu3P+C10H21 | Br− | 2.56 | 49.78 |
| 5b | BF4− | 2.29 | 49.08 | |
| 6a | t-Bu3P+C12H25 | Br− | 2.59 | 50.06 |
| 6b | BF4− | 2.27 | 49.03 | |
| 7a | t-Bu3P+C14H29 | Br− | 2.68 | 50.01 |
| 7b | BF4− | 2.27 | 49.08 | |
| 8a | t-Bu3P+C16H33 | Br− | 2.65 | 49.53 |
| 8b | BF4− | 2.29 | 49.07 | |
| 9a | t-Bu3P+C18H37 | Br− | 2.62 | 50.12 |
| 9b | BF4− | 2.29 | 49.08 | |
| 10a | t-Bu3P+C20H41 | Br− | 2.54 | 49.51 |
| 10b | BF4− | 2.33 | 49.11 | |
| Entry | Phosphonium Salt | The Average Size of PdNPs, nm | The Average Size of PdNPs after Suzuki Reaction, nm |
|---|---|---|---|
| 1 | 1b | 6.43 ± 2.65 | 5.96 ± 2.08 |
| 2 | 2b | 3.69 ± 1.35 | 4.25 ± 2.21 |
| 3 | 3b | not observed | 3.57 ± 2.35 |
| 4 | 4b | 3.72 ± 1.13 | not observed |
| 5 | 5b | 2.78 ± 0.87 | 4.23 ± 1.36 |
| 6 | 6b | 2.01 ± 0.72 | 3.57 ± 1.62 |
| 7 | 7b | 2.56 ± 0.73 | 2.67 ± 0.83 |
| 8 | 8b | 3.11 ± 1.08 | 3.59 ± 1.27 |
| 9 | 9b | 2.81 ± 0.80 | 2.15 ± 0.66 |
| 10 | 10b | 3.62 ± 1.57 | 4.59 ± 2.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arkhipova, D.M.; Ermolaev, V.V.; Miluykov, V.A.; Gubaidullin, A.T.; Islamov, D.R.; Kataeva, O.N.; Ananikov, V.P. Sterically Hindered Phosphonium Salts: Structure, Properties and Palladium Nanoparticle Stabilization. Nanomaterials 2020, 10, 2457. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10122457
Arkhipova DM, Ermolaev VV, Miluykov VA, Gubaidullin AT, Islamov DR, Kataeva ON, Ananikov VP. Sterically Hindered Phosphonium Salts: Structure, Properties and Palladium Nanoparticle Stabilization. Nanomaterials. 2020; 10(12):2457. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10122457
Chicago/Turabian StyleArkhipova, Daria M., Vadim V. Ermolaev, Vasily A. Miluykov, Aidar T. Gubaidullin, Daut R. Islamov, Olga N. Kataeva, and Valentine P. Ananikov. 2020. "Sterically Hindered Phosphonium Salts: Structure, Properties and Palladium Nanoparticle Stabilization" Nanomaterials 10, no. 12: 2457. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10122457