Formation of Nanostructured Carbon from [Ni(NH3)6]3[Fe(CN)6]2
Abstract
:1. Introduction
2. Materials and Methods
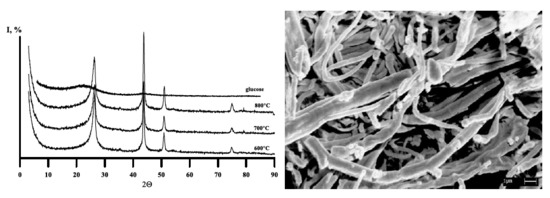
3. Results and Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Korenev, S.V.; Venediktov, A.B.; Shubin, Y.V.; Gromilov, S.A.; Yusenko, K.V. Synthesis and Structure of Binary Complexes of Platinum Group Metals - Precursors of Metallic Materials. J. Struct. Chem. 2003, 44, 46–59. [Google Scholar] [CrossRef]
- Shubin, Y.V.; Korenev, S.V. Formation of Nanosized Bimetallic Particles Based on Noble Metals. Catal. Ind. 2010, 2, 20–25. [Google Scholar] [CrossRef]
- Zadesenets, A.V.; Garkul, I.A.; Filatov, E.Y.; Plyusnin, P.E.; Filippov, T.N.; Asanova, T.I.; Korolkov, I.V.; Baidina, I.A.; Asanov, I.P.; Korenev, S.V. Oxalato complexes of Pd(II) with Co(II) and Ni(II) as single-source precursors for bimetallic nanoalloys. J. Therm. Anal. Calorim. 2019, 138, 111–121. [Google Scholar] [CrossRef]
- Pechenyuk, S.I.; Domonov, D.P.; Shimkin, A.A.; Ivanov, Y.V. Thermal decomposition of iron cyano-complexes in an inert atmosphere. Russ. Chem. Bull. 2015, 64, 322–328. [Google Scholar] [CrossRef]
- Pechenyuk, S.I.; Domonov, D.P.; Shimkin, A.A.; Semushina, Y.P.; Ivanov, Y.V. Thermal behavior of binary complex compounds containing the hexacyanoferrate anion. Russ. J. Gen. Chem. 2017, 87, 2212–2223. [Google Scholar] [CrossRef]
- Ng, C.W.; Ding, J.; Shi, Y.; Gan, L.M. Structure and magnetic properties of copper(II) hexacyanoferrate(III) copounds. J. Phys. and Chem. Solids 2001, 62, 767–775. [Google Scholar] [CrossRef]
- Ng, C.W.; Ding, J.; Wang, L.; Gan, L.M.; Quek, C.H. Thermal-Induced Microstructural Changes of Nickel-Iron Cyanide. J. Pys. Chem. A 2000, 104, 8814–8822. [Google Scholar] [CrossRef]
- Ng, C.W.; Ding, J.; Gan, L.M. Microstructural Changes Induced by Thermal Treatment of Cobalt(II) Hexacyanoferrate (III) Compound. J. Solid State Chem. 2001, 156, 400–407. [Google Scholar] [CrossRef]
- Song, Z.; Liu, X.; Sun, X.; Li, Y.; Nie, X.; Tang, W.; Yu, R.; Shui, J. Alginate-templated synthesis of CoFe/carbon fiber composite and the effect of hierarchically porous structure on electromagnetic wave absorption performance. Carbon 2019, 151, 36–45. [Google Scholar] [CrossRef]
- Ye, F.; Song, Q.; Zhang, Z.C.; Li, W.; Zhang, S.Y.; Yin, X.W.; Zhou, Y.; Tao, H.; Liu, Y.; Cheng, L.; et al. Direct growth of edge-rich graphene with tunable dielectric properties in porous Si3N4 ceramic for broadband high-performance microwave absorption. Adv. Funct. Mater. 2018, 28, 1707205. [Google Scholar] [CrossRef]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Man Hong, S.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pechenyuk, S.I.; Domonov, D.P.; Rogachev, D.L.; Belyaevskii, A.T. Anion effect on the thermolysis of double complexes [Co(NH3)6][Fe(CN)6] and [Co(NH3)6]4[Fe(CN)6]3. Russ. J. Inorg. Chem. 2007, 52, 1033–1038. [Google Scholar] [CrossRef]
- Pechenyuk, S.I.; Domonov, D.P.; Avedisyan, A.A.; Ikorskii, S.V. Conversions of coordinated ligands by reducing thermolysis of some double complex compounds. Russ. Inorg. Chem. 2010, 55, 734–738. [Google Scholar] [CrossRef]
- Brauer, G. Handbuch der Präparativen Anorganischen Chemie: In Drei Bänden; Ferdinand Enke: Stuttgart, Germany, 1978; 2113p. [Google Scholar]
- JCPDS-JCDD Card. Newtown Square (PA, USA): International Centre for Diffraction Data; JCPDS-JCDD Card: Newtown Square, PA, USA, 2002. [Google Scholar]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic computing system JANA2006: General Features. Z. Kristallogr. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; Part A: Theory and Applications in Inorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2009; 432p. [Google Scholar]
- Gravereau, P.; Garnier, E. Structure de la phase cubique de l’hexacyanoferrate(III) de zinc: Zn3[Fe(CN)6]2.nH2O. Acta Crystallogr. Sect. C 1984, 40, 1306–1309. [Google Scholar] [CrossRef]
- Makhorin, K.E.; Zayats, N.N.; Donchak, S.S. Analiz derivatogramm okislennogo i vspuchennogo grafita. Khim. tekhnologiya. 1990, 3, 44–47. (In Russian) [Google Scholar]
- Kalashnikova, M.Y. Derivatographic study of thermally expanded graphite products. Vestnik PGTU. Problems of modern materials and technologies. Permian 2001, 7, 82–91. (In Russian) [Google Scholar]
- Vazquez-Santos, M.B.; Geissler, E.; Laszlo, K.; Rouzaud, J.N.; Martinez-Alonso, A.; Tascon, J.M.D. Comparative XRD, Raman and TEM Study on graphition of PBO-derived Carbon Fibers. J. Phys. Chem. C 2012, 116, 257–268. [Google Scholar] [CrossRef]
- Domrachev, G.A.; Lazarev, A.I.; Kaverin, B.S.; Egorochkin, A.N.; Ob”edkov, A.M.; Domracheva, E.G.; Markin, G.V.; Huipe Nava, E.; Sorokin, A.A.; Suvorova, O.N.; et al. The Role of Carbon and Metal in Self-Assembly of the Iron–Carbon System at Various Component Ratios. Phys. Solid State. 2004, 46, 1969–1983. [Google Scholar] [CrossRef]







| Thermolysis Temperature, °C | Absorption Lines, cm−1 |
|---|---|
| starting [Ni(NH3)4]3[Fe(CN)6]2 | 3374, 3242 ν(NH3); 2169, 2095 ν(C≅N); 1613 δd(NH3); 1415 δd(NH4); 1186 δs(NH3); 594 ρr (NH3); 467 ν(M-N) 1 |
| 200 | 3367, 3244 ν(NH3); 2163, 2097 ν(C≅N); 1610 δd(NH3); 1414 δd(NH4); 596 ρr(NH3); 444 ν(M-N) |
| 275 | 3261 ν(NH3); 2162, 2097, 2055 ν(C≅N); 1607δd(NH3); 1414 δd(NH4); 1258 δs(NH3); 596 ρr(NH3); 445 ν(M-N) |
| 360 | 3386, 3156 ν(NH3); 2167, 2059 ν(C≅N); 1608 δd(NH3); 1413 δd(NH4); 1254 δs(NH3); 580 ρr(NH3); 457 ν(Fe-N) |
| T1, °C | Residue, wt.% | M.m.2 | Composition, wt.% | Released Ammonia, mol | Ssp3, m2/g | Description of Samples | ||
|---|---|---|---|---|---|---|---|---|
| Ni | Fe | C | ||||||
| starting | 100 | 905.7 803.7 | 19.44 21.8 | 12.3 14.0 | 15.9/100 17.7/100 | – – | – | Ni3Fe2C12H54N30 = [Ni(NH3)6]3[Fe(CN)6]2 Ni3Fe2C12H36N24 = [Ni(NH3)4]3[Fe(CN)6]2 |
| 200 | 83.9 79 | 736 660 | 23.9 26.3 | 15.1 17.1 | 19.7 20.9 | – 2.7 | – | Ni3Fe2C12H24N20 = [Ni3(NH3)8][Fe2(CN)12][4] Ni3Fe2C11.5H12N15.5 = [Ni3(NH3)4][Fe2(CN)11.5] |
| 275 | 79 74 | 736 630 | 23.9 28.1 | 15.0 18.0 | 19.1 21.2 | – 4.4 | – – | Ni3Fe2C12H24N20 = [Ni3(NH3)8][Fe(CN)6]2 Ni3Fe2C11.5H9N15.5 = [Ni3(NH3)3][Fe2(CN)11] |
| 360 | 66.3 | 600 | 29.7 31.4 | 18.2 18.7 | 20.4/85.1 | 5.0 | 170 | Green residue Ni3Fe2(CN)10.4 |
| 600 | 41.08 | 372 | 47.54 | 29.56 | 23.2 | – | 27.71 | Ni3Fe2C7.3 |
| 650 | 44.3 | 401 (375) | – | – | 23.9/66.6 | 6.8 | 40.5 | Black, loose, Ni3Fe2C7N2 Sharp lines of Ni3Fe |
| 650 | 44.4 | 402 (375) | 49.0 | 29.8 | 22.0/61.4 | 6.5 | 43.8 | |
| 700 | 39.9 | 361 | 48.80 | 31.54 | 18.7 | – | 72.73 | Ni3Fe2C5.5N0.5 |
| 800 | 45 41.6 | 400 377 | 44.4 48.43 | 28.5 31.23 | 21.6 23,6 | 6.2 – | 78,54 77.52 | Ni3Fe2C7N2 Ni3Fe2C7N0.5 |
| 1000 | 46.2 | 400 | 44.4 | 28.1 | 21.0 | – | – | Ni3Fe2C7 [4] |
| Thermolysis Temperature, °C | Carbon Yield, g/g | Ssp, m2/g | d002, Å | Crystallite Size, nm | Content of Metals in Carbon, wt.% | |
|---|---|---|---|---|---|---|
| Ni | Fe | |||||
| 600 | 0.30 | 224 | 0.341 | 14 | – | – |
| 650 | 0.27 0.24 | 148 226 | 0.337 0.335 | 36 38 | 4.2 – | 3.4 – |
| 700 | 0.28 | 276 | 0.341 | 14 | 8.03 | 6.77 |
| 800 | 0.33 0.30 | 230 209 | 0.343 0.341 | 40 42 | 10.4 8.44 | 7.5 6.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domonov, D.P.; Pechenyuk, S.I.; Belyaevskii, A.T.; Yusenko, K.V. Formation of Nanostructured Carbon from [Ni(NH3)6]3[Fe(CN)6]2. Nanomaterials 2020, 10, 389. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10020389
Domonov DP, Pechenyuk SI, Belyaevskii AT, Yusenko KV. Formation of Nanostructured Carbon from [Ni(NH3)6]3[Fe(CN)6]2. Nanomaterials. 2020; 10(2):389. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10020389
Chicago/Turabian StyleDomonov, Denis P., Sophiya I. Pechenyuk, Alexander T. Belyaevskii, and Kirill V. Yusenko. 2020. "Formation of Nanostructured Carbon from [Ni(NH3)6]3[Fe(CN)6]2" Nanomaterials 10, no. 2: 389. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10020389