Removal and Recovery of Methyl Tertiary Butyl Ether (MTBE) from Water Using Carbon Nanotube and Graphene Oxide Immobilized Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. CNIM-f and GOIM Fabrication and Characterization
2.3. Experimental Setup
2.4. Experimental Procedure
3. Results and Discussion
3.1. SGMD Performance Using GOIM, CNIM-f and PTFE Membrane
3.2. Mass Transfer Coefficients
3.3. Membrane Stability
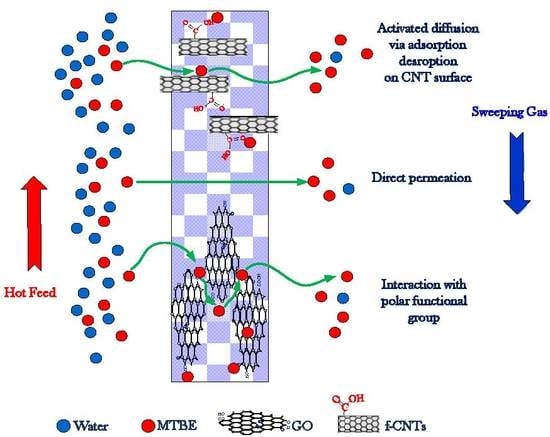
4. Proposed Mechanism
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Danmaliki, G.I.; Shamsuddeen, A.A.; Usman, B.J. The effect of temperature, turbulence, and Ph on the solubility of MTBE. Eur. J. Earth Environ. 2016, 3, 31–39. [Google Scholar]
- Rosell, M.; Lacorte, S.; Barceló, D. Analysis, occurrence and fate of MTBE in the aquatic environment over the past decade. TrAC Trends Anal. Chem. 2006, 25, 1016–1029. [Google Scholar] [CrossRef]
- Dale, M.S.; Koch, B.; Losee, R.F.; Crofts, E.W.; Davis, M.K. MTBE in Southern California water. J. Am. Water Work. Assoc. 2000, 92, 42–51. [Google Scholar] [CrossRef]
- Gullick, R.W.; LeChevallier, M.W. Occurrence of MTBE in drinking water sources. J. Am. Water Work. Assoc. 2000, 92, 100–113. [Google Scholar] [CrossRef]
- Toran, L.; Lipka, C.; Baehr, A.; Reilly, T.; Baker, R. Seasonal and daily variations in concentrations of methyl-tertiary-butyl ether (MTBE) at Cranberry Lake, New Jersey. Water Res. 2003, 37, 3756–3766. [Google Scholar] [CrossRef] [Green Version]
- Facetti, J.F.; Nunez, R.; Gomez, C.; Ojeda, J.; Bernal, C.; Leon-Ovelar, R.; Carvallo, F. Methyl tert-butyl ether (MtBE) in deep wells of the Patiño Aquifer, Paraguay: A preliminary characterization. Sci. Total Environ. 2019, 647, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.C.; Haderlein, S.B.; Pfister, R.; Forster, R. Occurrence and fate modeling of MTBE and BTEX compounds in a Swiss Lake used as drinking water supply. Water Res. 2004, 38, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Morgenroth, E.; Arvin, E. The European Perspective of MTBE as an Oxygenate in Fuels. In Managing for Healthy Ecosystems; 1447: ROUTLEDGE in association with GSE Research; CRC: Boca Raton, FL, USA, 2003; pp. 1447–1458. [Google Scholar]
- Zhang, L.; Qin, J.; Zhang, Z.; Li, Q.; Huang, J.; Peng, X.; Qing, L.; Liang, G.; Liang, L.; Huang, Y.; et al. Concentrations and potential health risks of methyl tertiary-butyl ether (MTBE) in air and drinking water from Nanning, South China. Sci. Total Environ. 2016, 541, 1348–1354. [Google Scholar] [CrossRef]
- Levchuk, I.; Bhatnagar, A.; Sillanpää, M. Overview of technologies for removal of methyl tert-butyl ether (MTBE) from water. Sci. Total Environ. 2014, 476, 415–433. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W. Removal of hazardous volatile organic compounds from water by vacuum pervaporation with hydrophobic ceramic membranes. J. Membr. Sci. 2015, 474, 11–19. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, Q.; Lv, R.; Soyekwo, F.; Zhu, A.; Liu, Q. Highly efficient polymer–MOF nanocomposite membrane for pervaporation separation of water/methanol/MTBE ternary mixture. Chem. Eng. Res. Des. 2017, 117, 688–697. [Google Scholar] [CrossRef]
- Keller, A.A.; Sandall, O.C.; Rinker, R.G.; Mitani, M.M.; Bierwagen, B.; Snodgrass, M.J. Cost and performance evaluation of treatment technologies for MTBE-contaminated water. Health Environ. Assess. MTBE 1998, 4, 1–41. [Google Scholar]
- Hung, H.-W.; Lin, T.-F. Adsorption of MTBE from contaminated water by carbonaceous resins and mordenite zeolite. J. Hazard. Mater. 2006, 135, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.; Adams, C.; Kekobad, J. Treatment of MTBE by air stripping, carbon adsorption, and advanced oxidation: Technical and economic comparison for five groundwaters. Water Res. 2004, 38, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Vane, L.M.; Alvarez, F.R.; Mullins, B. Removal of methyl tert-butyl ether from water by pervaporation: Bench-and pilot-scale evaluations. Environ. Sci. Technol. 2001, 35, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, W.; Cohen, Y. Removal of methyl tert-butyl ether from water by pervaporation using ceramic-supported polymer membranes. J. Membr. Sci. 2004, 229, 27–32. [Google Scholar] [CrossRef]
- Karp, J.R.; Hamerski, F.; Silva, V.R. Supported silk fibroin/poly (vinyl alcohol) membrane blends: Structure, properties, and ethanol dehydration by pervaporation. Polym. Eng. Sci. 2018, 58, 1879–1887. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Shirazi, A.; Mahdi, M.; Kargari, A. A review on applications of membrane distillation (MD) process for wastewater treatment. J. Membr. Sci. Res. 2015, 1, 101–112. [Google Scholar]
- Gupta, O.; Roy, S.; Mitra, S. Enhanced membrane distillation of organic solvents from their aqueous mixtures using a carbon nanotube immobilized membrane. J. Membr. Sci. 2018, 568, 134–140. [Google Scholar] [CrossRef]
- Roy, S.; Ragunath, S. Emerging membrane technologies for water and energy sustainability: Future prospects, constraints and challenges. Energies 2018, 11, 2997. [Google Scholar] [CrossRef] [Green Version]
- Intrchom, W.; Roy, S.; Mitra, S. Functionalized carbon nanotube immobilized membrane for low temperature ammonia removal via membrane distillation. Sep. Purif. Technol. 2020, 235, 116188. [Google Scholar] [CrossRef]
- Darling, S.B. Perspective: Interfacial materials at the interface of energy and water. J. Appl. Phys. 2018, 124, 030901. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Membrane synthesis for membrane distillation: A review. Sep. Purif. Technol. 2017, 182, 36–51. [Google Scholar] [CrossRef]
- Jung, J.; Shin, Y.; Choi, Y.-J.; Sohn, J.; Lee, S.; An, K. Hydrophobic surface modification of membrane distillation (MD) membranes using water-repelling polymer based on urethane rubber. Desalin. Water Treat. 2016, 57, 10031–10041. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, B.; Li, X.-M.; Wang, Z.; He, B.-Q.; He, T.; Jiang, B. CF4 plasma surface modification of asymmetric hydrophilic polyethersulfone membranes for direct contact membrane distillation. J. Membr. Sci. 2012, 407, 164–175. [Google Scholar] [CrossRef]
- Biniaz, P.; Torabi Ardekani, N.; Makarem, M.A.; Rahimpour, M.R. Water and Wastewater Treatment Systems by Novel Integrated Membrane Distillation (MD). ChemEngineering 2019, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Gethard, K.; Sae-Khow, O.; Mitra, S. Carbon nanotube enhanced membrane distillation for simultaneous generation of pure water and concentrating pharmaceutical waste. Sep. Purif. Technol. 2012, 90, 239–245. [Google Scholar] [CrossRef]
- Roy, S.; Bhadra, M.; Mitra, S. Enhanced desalination via functionalized carbon nanotube immobilized membrane in direct contact membrane distillation. Sep. Purif. Technol. 2014, 136, 58–65. [Google Scholar] [CrossRef]
- Bhadra, M.; Roy, S.; Mitra, S. Desalination across a graphene oxide membrane via direct contact membrane distillation. Desalination 2016, 378, 37–43. [Google Scholar] [CrossRef]
- Bhadra, M.; Roy, S.; Mitra, S. Enhanced desalination using carboxylated carbon nanotube immobilized membranes. Sep. Purif. Technol. 2013, 120, 373–377. [Google Scholar] [CrossRef]
- Intrchom, W.; Roy, S.; Humoud, M.S.; Mitra, S. Immobilization of graphene oxide on the permeate side of a membrane distillation membrane to enhance flux. Membranes 2018, 8, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandini, S.; Saavedra, A.; Sarti, G.C. Vacuum membrane distillation: Experiments and modeling. Aiche J. 1997, 43, 398–408. [Google Scholar] [CrossRef]
- Sarti, G.; Gostoli, C.; Bandini, S. Extraction of organic components from aqueous streams by vacuum membrane distillation. J. Membr. Sci. 1993, 80, 21–33. [Google Scholar] [CrossRef]
- Chen, Y.; Mitra, S. Fast microwave-assisted purification, functionalization and dispersion of multi-walled carbon nanotubes. J. Nanosci. Nanotechnol. 2008, 8, 5770–5775. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Vicente, C.; André, P.; Ferreira, R. Simple measurement of surface free energy using a web cam. Rev. Bras. Ensino Física 2012, 34, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Sirkar, K.K. Studies in vacuum membrane distillation with flat membranes. J. Membr. Sci. 2017, 523, 225–234. [Google Scholar] [CrossRef]
- Firsov, S.; Zhbankov, G.; Bakhramov, M.; Abdukadyrov, A.; Gafurov, A. Raman spectra and structure of polytetrafluoroethylene subjected to elastic deformation grinding. J. Appl. Spectrosc. 1993, 59, 644–647. [Google Scholar] [CrossRef]
- Dresselhaus, M.; Jorio, A.; Saito, R. Characterizing graphene, graphite, and carbon nanotubes by Raman spectroscopy. Annu. Rev. Condens. Matter Phys. 2010, 1, 89–108. [Google Scholar] [CrossRef]
- Golebiewski, J.; Galeski, A. Thermal stability of nanoclay polypropylene composites by simultaneous DSC and TGA. Compos. Sci. Technol. 2007, 67, 3442–3447. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Yu, H.; Xing, D.; Lu, W.; Shao, Z.; Yi, B. Preparation and characterization of PTFE based composite anion exchange membranes for alkaline fuel cells. J. Membr. Sci. 2012, 421, 311–317. [Google Scholar] [CrossRef]
- Su, S.; Xu, Y.; Wilkie, C. Thermal degradation of polymer–carbon nanotube composites. In Polymer–Carbon Nanotube Composites; Elsevier: Amsterdam, The Netherlands, 2011; pp. 482–510. [Google Scholar]
- Nguyen, B.D.; Ngo, T.K.; Bui, T.H.; Pham, D.K.; Dinh, X.L.; Nguyen, P.T. The impact of graphene oxide particles on viscosity stabilization for diluted polymer solutions using in enhanced oil recovery at HTHP offshore reservoirs. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 6, 015012. [Google Scholar] [CrossRef]
- Kavinkumar, T.; Manivannan, S. Synthesis, characterization and gas sensing properties of graphene oxide-multiwalled carbon nanotube composite. J. Mater. Sci. Technol. 2016, 32, 626–632. [Google Scholar] [CrossRef]
- Saffarini, R.B.; Mansoor, B.; Thomas, R.; Arafat, H.A. Effect of temperature-dependent microstructure evolution on pore wetting in PTFE membranes under membrane distillation conditions. J. Membr. Sci. 2013, 429, 282–294. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef]
- Street, A.; Sustich, R.; Duncan, J.; Savage, N. Nanotechnology Applications for Clean Water: Solutions for Improving Water Quality; William Andrew: New York, NY, USA, 2014. [Google Scholar]
- Lee, C.H.; Hong, W.H. Effect of operating variables on the flux and selectivity in sweep gas membrane distillation for dilute aqueous isopropanol. J. Membr. Sci. 2001, 188, 79–86. [Google Scholar] [CrossRef]
- Charfi, K.; Khayet, M.; Safi, M.J. Numerical simulation and experimental studies on heat and mass transfer using sweeping gas membrane distillation. Desalination 2010, 259, 84–96. [Google Scholar] [CrossRef]
- Feng, X.; Huang, R.Y. Estimation of activation energy for permeation in pervaporation processes. J. Membr. Sci. 1996, 118, 127–131. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Koter, S.; Kujawski, W. Highly efficient hydrophobic titania ceramic membranes for water desalination. ACS Appl. Mater. Interfaces 2014, 6, 14223–14230. [Google Scholar] [CrossRef]
- Feng, C.; Khulbe, K.; Tabe, S. Volatile organic compound removal by membrane gas stripping using electro-spun nanofiber membrane. Desalination 2012, 287, 98–102. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, B.; Fan, S.; Liu, J.; Qin, Y.; Jian, S.; Wang, Y.; Xiao, Z. Membrane distillation of butanol from aqueous solution with polytetrafluoroethylene membrane. Chem. Eng. Technol. 2020. [Google Scholar] [CrossRef]
- Ray, S.; Singha, N.; Ray, S. Removal of tetrahydrofuran (THF) from water by pervaporation using homo and blend polymeric membranes. Chem. Eng. J. 2009, 149, 153–161. [Google Scholar] [CrossRef]
- Ray, S.; Ray, S. Synthesis of highly methanol selective membranes for separation of methyl tertiary butyl ether (MTBE)–methanol mixtures by pervaporation. J. Membr. Sci. 2006, 278, 279–289. [Google Scholar] [CrossRef]
- Sweetman, M.J.; May, S.; Mebberson, N.; Pendleton, P.; Vasilev, K.; Plush, S.E.; Hayball, J.D. Activated carbon, carbon nanotubes and graphene: Materials and composites for advanced water purification. C J. Carbon Res. 2017, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- You, Y.; Sahajwalla, V.; Yoshimura, M.; Joshi, R.K. Graphene and graphene oxide for desalination. Nanoscale 2016, 8, 117–119. [Google Scholar] [CrossRef]
- Gethard, K.; Sae-Khow, O.; Mitra, S. Water desalination using carbon-nanotube-enhanced membrane distillation. ACS Appl. Mater. Interfaces 2011, 3, 110–114. [Google Scholar] [CrossRef]










| Temperature (°C) | Mass Transfer Coefficient (kg/m2 s Pa) × 10−6 | ||
|---|---|---|---|
| PTFE Membrane | GOIM | CNIM-f | |
| 21 | 2.19 ± 0.08 | 2.41 ± 0.08 | 2.51 ± 0.09 |
| 30 | 1.70 ± 0.02 | 1.77 ± 0.02 | 1.88 ± 0.03 |
| 40 | 1.29 ± 0.04 | 1.38 ± 0.04 | 1.45 ± 0.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intrchom, W.; Roy, S.; Mitra, S. Removal and Recovery of Methyl Tertiary Butyl Ether (MTBE) from Water Using Carbon Nanotube and Graphene Oxide Immobilized Membranes. Nanomaterials 2020, 10, 578. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10030578
Intrchom W, Roy S, Mitra S. Removal and Recovery of Methyl Tertiary Butyl Ether (MTBE) from Water Using Carbon Nanotube and Graphene Oxide Immobilized Membranes. Nanomaterials. 2020; 10(3):578. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10030578
Chicago/Turabian StyleIntrchom, Worawit, Sagar Roy, and Somenath Mitra. 2020. "Removal and Recovery of Methyl Tertiary Butyl Ether (MTBE) from Water Using Carbon Nanotube and Graphene Oxide Immobilized Membranes" Nanomaterials 10, no. 3: 578. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10030578