The Improvement of Nanoemulsion Stability and Antioxidation via Protein-Chlorogenic Acid-Dextran Conjugates as Emulsifiers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Conjugates
2.2.1. Native Proteins (NP)
2.2.2. Protein-Chlorogenic Acid Conjugates (PC)
2.2.3. Protein-Chlorogenic Acid-Dextran Conjugate (PCD)
2.3. The Conjugates Structural Analysis
2.3.1. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.3.2. Fourier Transform Infrared (FT-IR) Spectroscopy Measurement
2.3.3. Fluorescence Measurements
2.4. Interfacial Tensions (IT) Measurement
2.5. The Activities of Antioxidant
2.5.1. DPPH Radical Scavenging Activities
2.5.2. ABTS Radical Scavenging Activities
2.5.3. Iron Reduction Activities
2.6. Preparation of Nanoemulsions
2.7. Droplet Size and Zeta-Potential Measurements of Nanoemulsions
2.8. Apparent Viscosity Measurement of Nanoemulsions
2.9. The Stability Determination of Nanoemulsions
2.9.1. Storage Stability
2.9.2. Oxidative Stability
2.9.3. Thermal Stability
2.9.4. Freeze-Thaw Stability
2.10. Statistical Analysis
3. Results
3.1. Structural Analysis of PC and PCD Conjugates
3.1.1. SDS-PAGE Analysis
3.1.2. Fourier Transform Infrared (FT-IR) Analysis
3.1.3. Fluorescence Spectroscopy Analysis
3.2. Properties Analysis of PC and PCD Conjugates
3.2.1. Interfacial Tensions
3.2.2. Analysis of Antioxidant Activities
3.3. Characterization of PC and PCD Stabilized Nanoemulsion
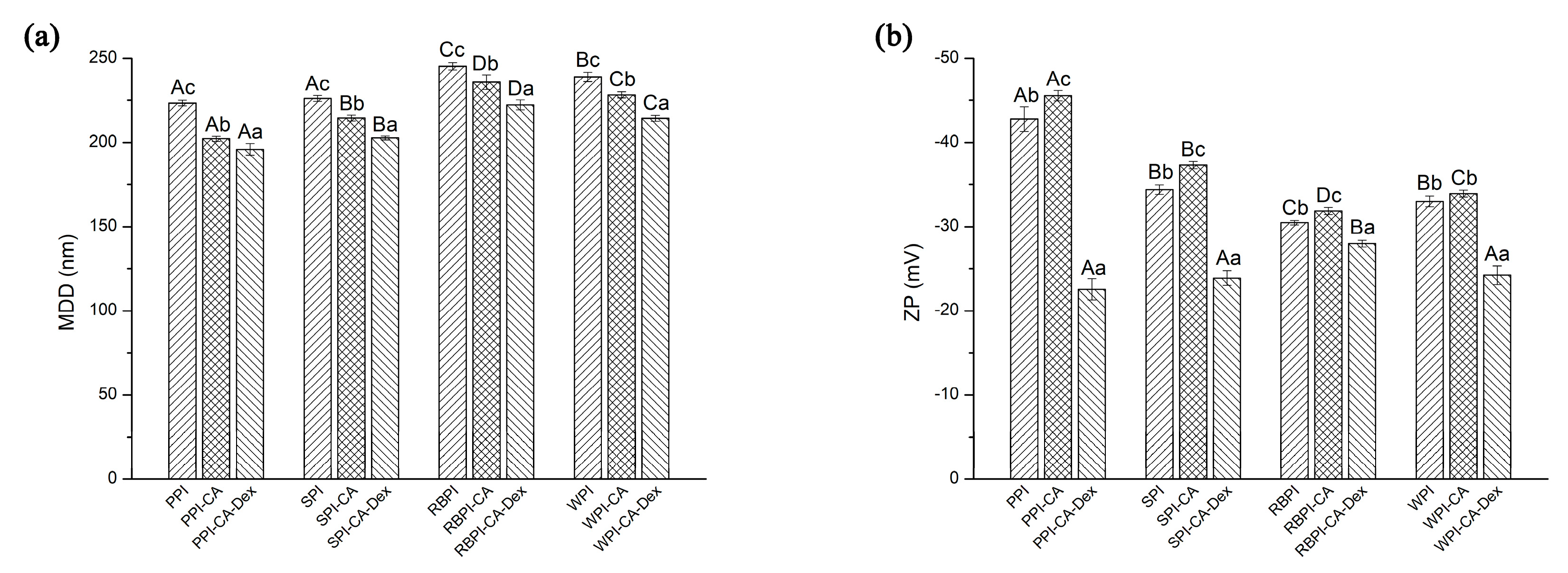
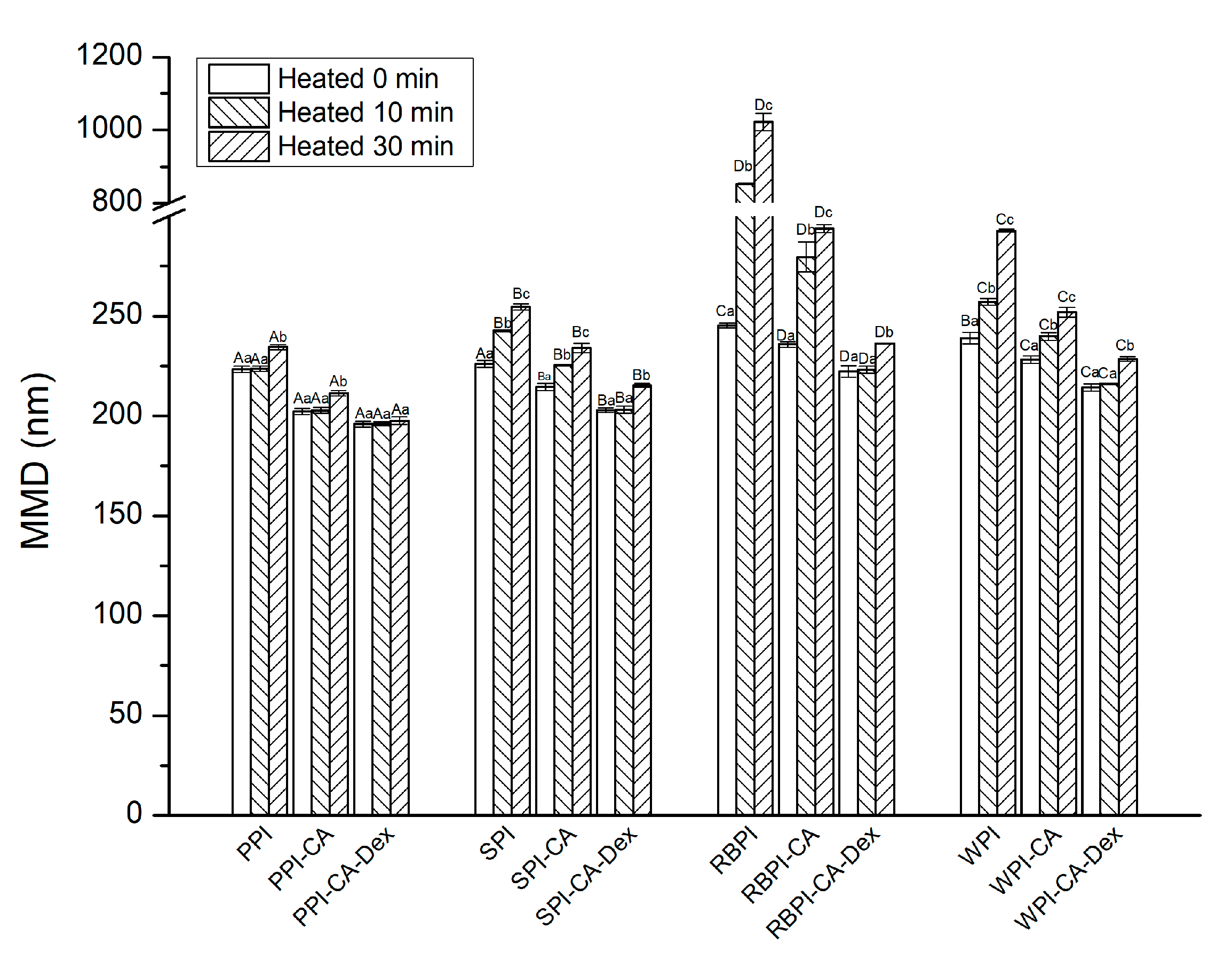
3.3.1. Mean Droplet Diameters and Zeta-Potential
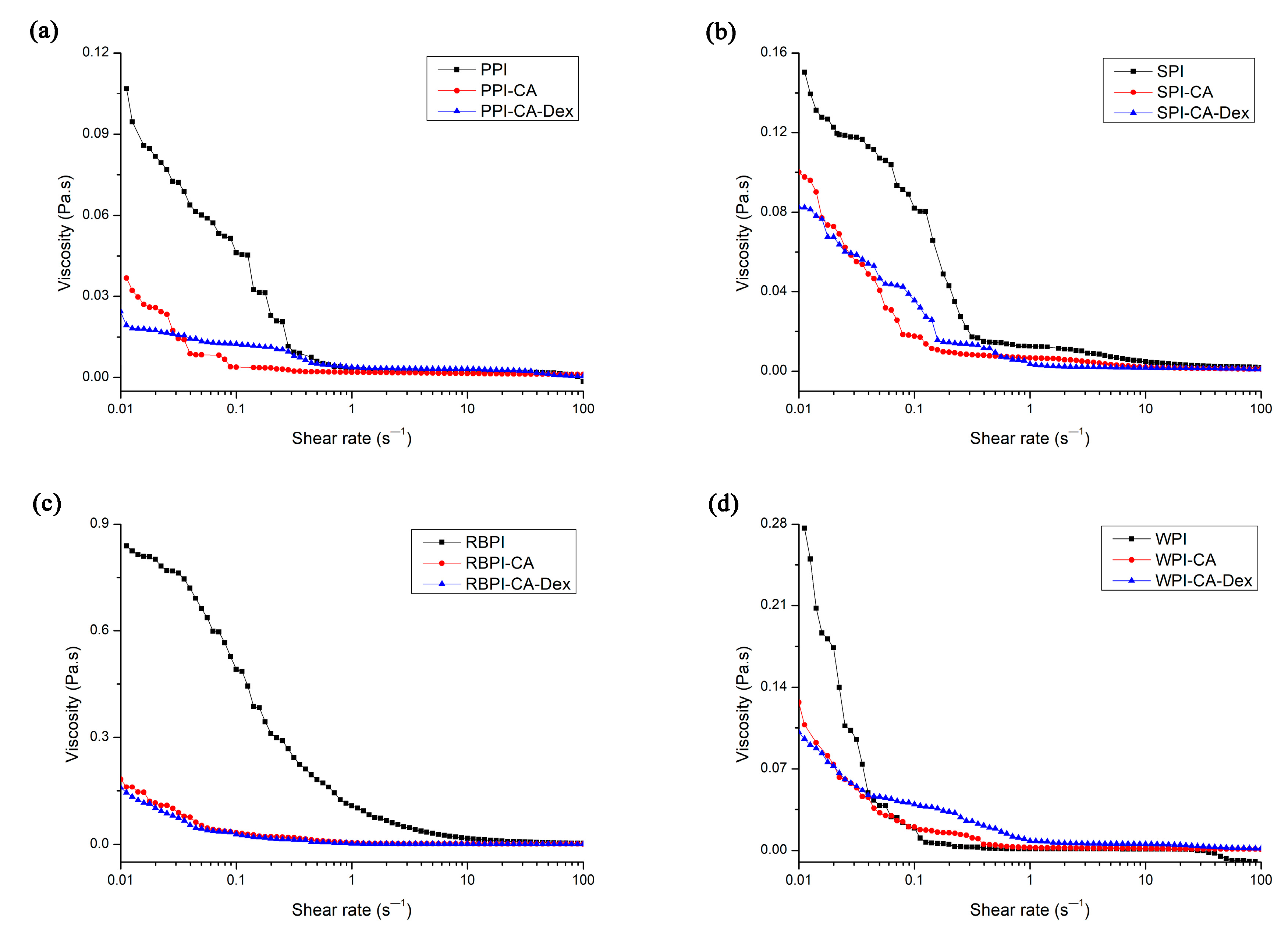
3.3.2. Apparent Viscosity of Nanoemulsion
3.4. Stability of PC, PCD Stabilized Nanoemulsion
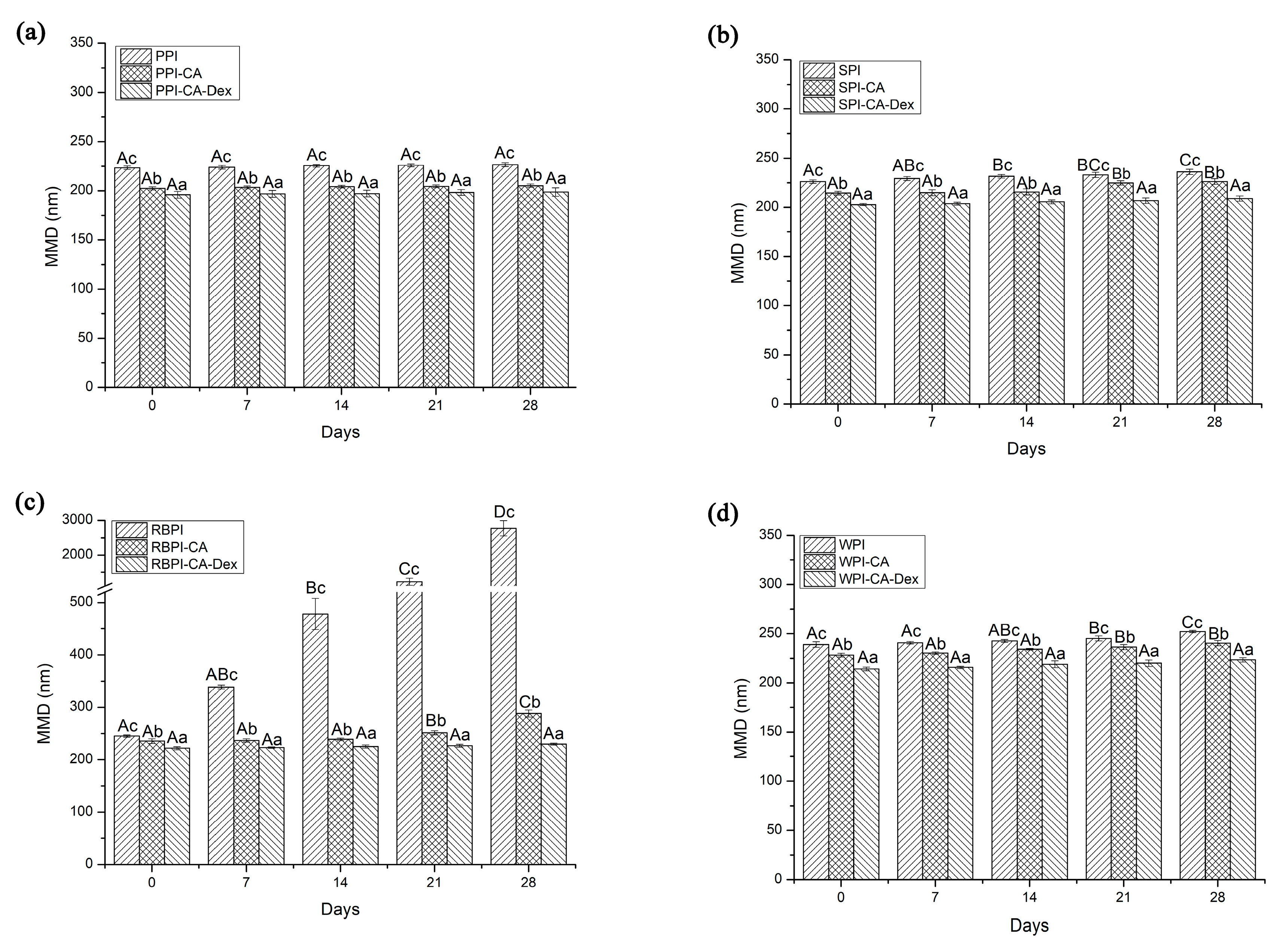
3.4.1. Storage Stability
3.4.2. Oxidation Stability
3.4.3. Thermal Stability
3.4.4. Freeze-Thaw Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-amino-di(2-ethyl-benzothiazoline sulphonic acid-6)ammonium salt |
| BHT | butylated hydroxytoluene |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl radical 2,2-Diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl |
| EGCG | epigallocatechin gallate |
| FTIR | Fourier Transform Infrared |
| IT | Interfacial Tensions |
| MDD | mean droplet diameters |
| PC | protein-chlorogenic acid |
| PCD | protein-chlorogenic acid-dextran |
| PPI | peanut protein isolate |
| RBPI | rice bran protein isolate |
| SDS-PAGE | Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis |
| SPI | soybean protein isolate |
| WPI | whey protein isolate |
References
- McClements, D.J. Advances in fabrication of emulsions with enhanced functionality using structural design principles. Curr. Opin. Colloid Interface 2012, 17, 235–245. [Google Scholar] [CrossRef]
- Li, Y.; Jin, H.; Sun, X.; Sun, J.; Liu, C.; Xu, J. Physicochemical properties and storage stability of food protein-stabilized nanoemulsions. Nanomaterials 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Wang, D.; Sun, C.; Gao, Y. Influence of polysaccharides on the physicochemical properties of lactoferrin-polyphenol conjugates coated β-carotene emulsions. Food Hydrocoll. 2016, 52, 661–669. [Google Scholar] [CrossRef]
- Jin, H.; Wang, X.; Chen, Z.; Li, Y.; Liu, C.; Xu, J. Fabrication of β-conglycinin-stabilized nanoemulsions via ultrasound process and influence of SDS and PEG 10000 co-emulsifiers on the physicochemical properties of nanoemulsions. Food Res. Int. 2018, 106, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Cortés, M.; Chevalier, L.; Dumay, E. Characteristics of submicron emulsions prepared by ultra-high pressure homogenisation: Effect of chilled or frozen storage. Food Hydrocoll. 2009, 23, 640–654. [Google Scholar] [CrossRef]
- Xu, J.; Mukherjee, D.; Chang, S.K.C. Physicochemical properties and storage stability of soybean protein nanoemulsions prepared by ultra-high pressure homogenization. Food Chem. 2018, 240, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, Y.; Ngadi, M. Binding of curcumin to β-lactoglobulin and its effect on antioxidant characteristics of curcumin. Food Chem. 2013, 141, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, Y.; Gao, L.; Zhang, Y.; Yi, J. Oxidative stability and, in vitro, digestion of menhaden oil emulsions with whey protein: Effects of EGCG conjugation and interfacial cross-linking. Food Chem. 2018, 265, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Ismail, H.; Hossam, S.; Sampson, A.; Chiranjib, B.; Petersen, S.V.; Pérez, B.; Guo, Z. Adding functionality to milk-based protein: Preparation, and physicochemical characterization of β-lactoglobulin-phenolic conjugates. Food Chem. 2018, 241, 281–289. [Google Scholar]
- Liu, F.; Sun, C.; Yang, W.; Yuan, F.; Gao, Y. Structural characterization and functional evaluation of lactoferrin-polyphenol conjugates formed by free-radical graft copolymerization. RSC Adv. 2015, 5, 15641–15651. [Google Scholar] [CrossRef]
- Diftis, N.; Biliaderis, C.; Kiosseoglou, V. Rheological properties and stability of model salad dressing emulsions prepared with a dry-heated soybean protein isolate-dextran mixture. Food Hydrocoll. 2005, 19, 1025–1031. [Google Scholar] [CrossRef]
- Boostani, S.; Aminlari, M.; Moosavi, M.; Niakosari, M.; Mesbahi, G. Fabrication and characterisation of soy protein isolate-grafted dextran biopolymer: A novel ingredient in spray-dried soy beverage formulation. Int. J. Biol. Macromol. 2017, 102, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Z.; Wang, X.; Gao, Y.; Zhao, Q.; Liu, C. Effect of enzymolysis and glycosylation on the curcumin nanoemulsions stabilized by β-conglycinin: Formation, stability and in vitro digestion. Int. J. Biol. Macromol. 2020, 142, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cui, S.; Gong, J.; Guo, Q.; Wang, Q.; Hua, Y. A soy protein-polysaccharides Maillard reaction product enhanced the physical stability of oil-in-water emulsions containing citral. Food Hydrocoll. 2015, 48, 155–164. [Google Scholar] [CrossRef]
- Liu, F.; Ma, C.; McClements, D.; Gao, Y. Development of polyphenol-protein-polysaccharide ternary complexes as emulsifiers for nutraceutical emulsions: Impact on formation, stability and bioaccessibility of β-carotene emulsions. Food Hydrocoll. 2016, 61, 578–588. [Google Scholar] [CrossRef]
- Sun, X.T.; Jin, H.; Li, Y.Y.; Feng, H.Y.; Liu, C.H.; Xu, J. The molecular properties of peanut protein: Impact of temperature, relative humidity and vacuum packaging during storage. Molecules 2018, 23, 2618. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, Z.; Handa, C.L.; Xu, J. Effects of ultrasound pre-treatment on the structure of β-conglycinin and glycinin and the antioxidant activity of their hydrolysates. Food Chem. 2017, 218, 165–172. [Google Scholar] [CrossRef]
- Shi, M.; Huang, L.Y.; Nie, N.; Ye, J.H.; Zheng, X.Q.; Lu, J.L.; Liang, Y.R. Binding of tea catechins to rice bran protein isolate: Interaction and protective effect during in vitro digestion. Food Res. Int. 2017, 93, 1–7. [Google Scholar] [CrossRef]
- Laemmli, B. Cleavage of structural proteins during assembly of head of bacteriophage-t4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Nucara, A. Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: A fourier transform infrared (FT-IR) spectroscopic study. Amino Acids 2012, 43, 911–921. [Google Scholar] [CrossRef]
- Bonomi, F.; Mora, G.; Pagani, M.; Iametti, S. Probing structural features of water-insoluble proteins by front-face fluorescence. Anal Biochem. 2004, 329, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, J.; Zhou, Y.; Huang, X.; Liu, F.; Pan, S.; Hu, H. Effect of high intensity ultrasound on physicochemical and functional properties of soybean glycinin at different ionic strengths. Innov. Food Sci. Emerg. 2016, 34, 205–213. [Google Scholar] [CrossRef]
- Hong, S.; Garcia, C.; Park, S.; Shin, G.; Kim, J. Retardation of curcumin degradation under various storage conditions via turmeric extract-loaded nanoemulsion system. LWT Food Sci. Technol. 2019, 100, 175–182. [Google Scholar] [CrossRef]
- Nejadmansouri, M.; Hosseini, S.; Niakosari, M.; Yousefi, G.; Golmakani, M. Physicochemical properties and storage stability of ultrasound-mediated WPI-stabilized fish oil nanoemulsions. Food Hydrocoll. 2016, 61, 801–811. [Google Scholar] [CrossRef]
- Liu, F.; Wang, D.; Sun, C.; McClements, D.; Gao, Y. Utilization of interfacial engineering to improve physicochemical stability of β-carotene emulsions: Multilayer coatings formed using protein and protein–polyphenol conjugates. Food Chem. 2016, 205, 129–139. [Google Scholar] [CrossRef]
- Zhao, J.; Xiang, J.; Wei, T.; Yuan, F.; Gao, Y. Influence of environmental stresses on the physicochemical stability of orange oil bilayer emulsions coated by lactoferrin–soybean soluble polysaccharides and lactoferrin–beet pectin. Food Res. Int. 2014, 66, 216–227. [Google Scholar] [CrossRef]
- You, J.; Luo, Y.; Wu, J. Conjugation of ovotransferrin with catechin shows improved antioxidant activity. J. Agric. Food Chem. 2014, 62, 2581–2587. [Google Scholar] [CrossRef]
- Feng, J.; Cai, H.; Wang, H.; Li, C.; Liu, S. Improved oxidative stability of fish oil emulsion by grafted ovalbumin-catechin conjugates. Food Chem. 2017, 241, 60–69. [Google Scholar] [CrossRef]
- Li, C.; Xue, H.; Chen, Z.; Ding, Q.; Wang, X. Comparative studies on the physicochemical properties of peanut protein isolate–polysaccharide conjugates prepared by ultrasonic treatment or classical heating. Food Res. Int. 2014, 57, 1–7. [Google Scholar] [CrossRef]
- Mu, L.; Zhao, M.; Yang, B.; Zhao, H.; Cui, C.; Zhao, Q. Effect of ultrasonic treatment on the graft reaction between soy protein isolate and gum acacia and on the physicochemical properties of conjugates. J. Agric. Food Chem. 2010, 58, 4494–4499. [Google Scholar] [CrossRef] [PubMed]
- Sahihi, M.; Ghayeb, Y.; Bordbar, A. Fluorescence spectroscopic study on interaction of retinol with β-lactoglobulin in the presence of cetylpyridinium chloride. J. Spectrosc. 2012, 27, 27–34. [Google Scholar] [CrossRef]
- Jia, J.; Gao, X.; Hao, M.; Tang, L. Comparison of binding interaction between ß-lactoglobulin and three common polyphenols using multi-spectroscopy and modeling methods. Food Chem. 2017, 228, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Jin, H.; Gao, Y.; Zhu, X.; Zhao, Q. The Effect of (−)-Epigallocatechin-3-Gallate Non-Covalent Interaction with the Glycosylated Protein on the Emulsion Property. Polymers 2019, 11, 1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Han, D.; Chen, Z.; Li, M.; Jin, H. Effect of glucose glycosylation following limited enzymatic hydrolysis on functional and conformational properties of black bean protein isolate. Eur. Food Res. Technol. 2018, 3, 1–10. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, Y. Chlorogenic acid-mediated gel formation of oxidatively stressed myofibrillar protein. Food Chem. 2015, 180, 235–243. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Mora, L.; Jemil, I.; Jridi, M.; Toldrá, F.; Nasri, R. Effect of ultrasound pretreatment and maillard reaction on structure and antioxidant properties of ultrafiltrated smooth-hound viscera proteins-sucrose conjugates. Food Chem. 2017, 230, 507–515. [Google Scholar] [CrossRef] [Green Version]
- Amine, C.; Dreher, J.; Helgason, T.; Tadros, T. Investigation of emulsifying properties and emulsion stability of plant and milk proteins using interfacial tension and interfacial elasticity. Food Hydrocoll. 2014, 39, 180–186. [Google Scholar] [CrossRef]
- Karefyllakis, D.; Altunkaya, S.; Berton, C. Physical bonding between sunflower proteins and phenols: Impact on interfacial properties. Food Hydrocoll. 2017, 73, 326–334. [Google Scholar] [CrossRef]
- Stéphanie, V.; Alphons, G.; Antonie, V.; Gerrit, V. Covalent interactions between proteins and oxidation products of caffeoylquinic acid (chlorogenic acid). J. Sci. Food Agric. 2007, 87, 13. [Google Scholar]
- Mustafa, R.; Hamid, A.; Mohamed, S. Total phenolic compounds, flavonoids, and radical scavenging activity of 21 selected tropical plants. J. Food Sci. 2010, 75, C28–C35. [Google Scholar] [CrossRef]
- Yi, J.; Zhang, Y.; Liang, R.; Zhong, F.; Ma, J. Beta-carotene chemical stability in nanoemulsions was improved by stabilized with beta-lactoglobulin–catechin conjugates through free radical method. J. Agric. Food Chem. 2015, 63, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, J.; Kong, B.; Jia, N.; Li, P. Antioxidant capacity of maillard reaction products formed by a porcine plasma protein hydrolysate-sugar model system as related to chemical characteristics. Food Sci. Biotechnol. 2014, 23, 33–41. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, J.; Zhao, Y. High intensity ultrasound assisted heating to improve solubility, antioxidant and antibacterial properties of chitosan-fructose maillard reaction products. LWT Food Sci. Technol. 2015, 60, 253–262. [Google Scholar] [CrossRef]
- Markman, G.; Livney, Y. Maillard-conjugate based core-shell co-assemblies for nanoencapsulation of hydrophobic nutraceuticals in clear beverages. Food Funct. 2011, 3, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Tagne, J.; Kakumanu, S.; Nicolosi, R. Nanoemulsion preparations of the anticancer drug dacarbazine significantly increase its efficacy in a xenograft mouse melanoma model. Mol. Pharm. 2008, 5, 1055–1063. [Google Scholar] [CrossRef]
- Fan, Y.; Yi, J.; Zhang, Y.; Wen, Z.; Zhao, L. Physicochemical stability and in vitro bioaccessibility of ß-carotene nanoemulsions stabilized with whey protein-dextran conjugates. Food Hydrocoll. 2017, 63, 256–264. [Google Scholar] [CrossRef]
- Sun, C.; Gunasekaran, S.; Richards, M. Effect of xanthan gum on physicochemical properties of whey protein isolate stabilized oil-in-water emulsions. Food Hydrocoll. 2007, 21, 555–564. [Google Scholar] [CrossRef]
- Delahaije, R.; Gruppen, H.; Nieuwenhuijzen, N.; Giuseppin, M.; Wierenga, P. Effect of glycation on the flocculation behavior of protein-stabilized oil-in-water emulsions. Langmuir 2013, 29, 15201–15208. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Liu, L.; Wei, Z.; Yuan, F.; Gao, Y. Physicochemical characterization of β-carotene emulsion stabilized by covalent complexes of α-lactalbumin with epigallocatechin gallate or chlorogenic acid. Food Chem. 2015, 173, 564–568. [Google Scholar] [CrossRef]
- Yi, J.; Lam, T.; Yokoyama, W.; Cheng, L.; Zhong, F. Cellular uptake of β-carotene from protein stabilized solid lipid nanoparticles prepared by homogenization–evaporation method. J. Agric. Food Chem. 2014, 62, 1096–1104. [Google Scholar] [CrossRef]
- Khalid, N.; Shu, G.; Holland, B.; Kobayashi, I.; Nakajima, M.; Barrow, C. Formulation and characterization of O/W nanoemulsions encapsulating high concentration of astaxanthin. Food Res. Int. 2017, 102, 364–371. [Google Scholar] [CrossRef] [PubMed]





| Samples | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Curl (%) |
|---|---|---|---|---|
| PPI | 12.01 ± 0.03 c | 30.17 ± 0.03 c | 41.26 ± 0.03 a | 16.56 ± 0.03 a |
| PPI-CA | 11.94 ± 0.03 b | 29.70 ± 0.02 b | 41.52 ± 0.02 b | 16.84 ± 0.01 b |
| PPI-CA-Dex | 11.05 ± 0.03 a | 28.65 ± 0.01 a | 41.94 ± 0.02 c | 18.36 ± 0.02 c |
| SPI | 12.84 ± 0.02 c | 31.44 ± 0.03 c | 38.99 ± 0.04 a | 16.73 ± 0.02 a |
| SPI-CA | 12.49 ± 0.02 b | 30.89 ± 0.02 b | 39.29 ± 0.01 b | 17.33 ± 0.01 b |
| SPI-CA-Dex | 11.60 ± 0.01 a | 30.71 ± 0.02 a | 39.71 ± 0.01 c | 17.98 ± 0.01 c |
| RBPI | 16.90 ± 0.01 c | 34.63 ± 0.03 c | 34.64 ± 0.02 a | 13.83 ± 0.02 a |
| RBPI-CA | 15.89 ± 0.02 b | 33.30 ± 0.02 b | 35.99 ± 0.01 b | 14.82 ± 0.02 b |
| RBPI-CA-Dex | 15.21 ± 0.01 a | 32.84 ± 0.01 a | 36.37 ± 0.01 c | 15.58 ± 0.02 c |
| WPI | 15.68 ± 0.02 c | 35.65 ± 0.02 c | 34.11 ± 0.04 a | 14.56 ± 0.03 a |
| WPI-CA | 14.82 ± 0.02 b | 35.07 ± 0.02 b | 34.68 ± 0.01 b | 15.43 ± 0.02 b |
| WPI-CA-Dex | 14.35 ± 0.01 a | 34.71 ± 0.02 a | 34.77 ± 0.02 c | 16.17 ± 0.02 c |
| Samples | DPPH Free Radical Scavenging Ability (%) | ABTS Free Radical Scavenging Ability (%) | Iron Reducing Ability (Abs) |
|---|---|---|---|
| PPI | 23.6 ± 0.3 a | 22.4 ± 0.3 a | 0.063 ± 0.002 a |
| PPI-CA | 62.2 ± 0.6 b | 95.7 ± 0.3 b | 0.340 ± 0.002 b |
| PPI-CA-Dex | 68.1 ± 0.5 c | 97.1 ± 0.3 c | 0.373 ± 0.002 c |
| SPI | 22.0 ± 0.3 a | 21.1 ± 0.2 a | 0.029 ± 0.002 a |
| SPI-CA | 55.7 ± 0.5 b | 83.4 ± 0.3 b | 0.265 ± 0.002 b |
| SPI-CA-Dex | 59.6 ± 0.2 c | 86.5 ± 0.4 c | 0.295 ± 0.001 c |
| RBPI | 18.7 ± 0.4 a | 17.7 ± 0.2 a | 0 a |
| RBPI-CA | 57.9 ± 0.4 b | 90.0 ± 0.2 b | 0.314 ± 0.002 b |
| RBPI-CA-Dex | 62.4 ± 0.3 c | 94.3 ± 0.3 c | 0.360 ± 0.002 c |
| WPI | 21.7 ± 0.2 a | 20.0 ± 0.3 a | 0 a |
| WPI-CA | 54.8 ± 0.6 b | 77.5 ± 0.4 b | 0.264 ± 0.002 b |
| WPI-CA-Dex | 58.6 ± 0.3 c | 80.8 ± 0.3 c | 0.292 ± 0.002 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Jin, H.; Yu, Y.; Sun, J.; Zheng, H.; Zhang, Y.; Xu, J.; Zhu, X. The Improvement of Nanoemulsion Stability and Antioxidation via Protein-Chlorogenic Acid-Dextran Conjugates as Emulsifiers. Nanomaterials 2020, 10, 1094. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10061094
Liu C, Jin H, Yu Y, Sun J, Zheng H, Zhang Y, Xu J, Zhu X. The Improvement of Nanoemulsion Stability and Antioxidation via Protein-Chlorogenic Acid-Dextran Conjugates as Emulsifiers. Nanomaterials. 2020; 10(6):1094. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10061094
Chicago/Turabian StyleLiu, Chang, Hua Jin, Yue Yu, Jingying Sun, Huanyu Zheng, Yan Zhang, Jing Xu, and Xiuqing Zhu. 2020. "The Improvement of Nanoemulsion Stability and Antioxidation via Protein-Chlorogenic Acid-Dextran Conjugates as Emulsifiers" Nanomaterials 10, no. 6: 1094. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10061094