3.1. Characterizations
Figure 2 shows the FTIR spectra and
Table 2 indicates the main absorption bands identified for each catalyst, according to the literature [
24,
25].
The asymmetric vibration on the SiO
4, identified at approximately 1220 cm
−1 (column A), and the asymmetric vibration between the zeolite tetrahedral and the oxygen atoms (TO, column B) were shifted to higher wavenumbers after the dealumination (increase of Si/Al ratio), in agreement with the literature [
26,
27]. However, no significant change was observed after the impregnation of Nb, within the experimental FTIR resolution (4 cm
−1).
In addition, the vibration corresponding to the Si–O bound (column C) shifted to higher wavenumbers for dealuminated sample (DB), which is also sensitive to Si/Al ratio [
27]. For the samples with Nb, if it had been incorporated into the zeolitic network, the wavenumber found would have been higher (960–970 cm
−1) [
28], but no significative shift was observed. Thus, we inferred that most of the impregnation of Nb occurred in the form of Nb
2O
5, outside the framework in all catalysts. The characteristic wavenumber of the symmetric vibration of the Si–O–Si connections of the zeolitic network (column D), and the vibrations that occurred in the six member zeolite rings (columns E, F and G) remained in the expected range, providing an indication that the dealumination did not compromise the zeolite structure [
24,
25,
26,
29].
The results of the XRD (
Figure 3) showed that the peaks at 2θ = 7.9° and 22.5° corresponded, respectively, to the planes (101) and (311), characteristic of the polymorph A of zeolite *BEA. The presence of these peaks in all catalysts is an indication that there were no significant changes with respect to the structure of the support, even after performing the dealumination procedure and niobium aqueous impregnation. Furthermore, as it was not possible to identify the presence of characteristic niobium oxide signals, it is also possible to infer that there might be a good dispersion of it on the support, or that there was the formation of amorphous species small enough that they could be not be detected by powder XRD [
23,
30]. We observed a decrease in the crystallinity content of the catalysts from 100% for HB, 87% for DB, 85% for 10%Nb-DB, 77% for 18%Nb-DB, and 85% for 25%Nb-DB, which did not significantly compromise the zeolite structure, as discussed previously.
Only the 25%Nb-DB catalyst showed the presence of three signals with relative low intensity at 2θ = 28.4°, 36.6°, and 46.2°, corresponding to the planes (180), (181), and (002) of the crystallographic T-phase of Nb
2O
5 (orthorhombic), respectively, which can be addressed to the incorporation of metal oxide outside the zeolitic framework [
24,
31,
32]. The difference in the relative peak intensities of pure T-Nb
2O
5 to 25%Nb-DB is related to the higher exposition of plane (180) on the supported one.
Table 3 presents the results of the textural analysis obtained from the isotherms for all catalysts (
Figure S2). Analyzing the obtained textural data (
Table 3), the specific surface area (S
BET) decreased after dealumination and Nb impregnation, which may indicate the obstruction of the pores by the presence of extra framework aluminum species (that cannot been completely removed after washing) and the presence of the supported material. This same trend was observed in the external surface area (S
Ext), except for the catalyst 18%Nb-DB, which showed a slight increase. The microporous area (S
Micro), and the volume of micropores (V
μ), decreased with the modifications exerted on the catalysts, but there was an increase in the diameter of the mesopores (D
Meso), due to dealumination, which can influence the access of the reactants to the active sites.
The signals obtained with the
27Al MAS NMR (
Figure 4) were integrated into two distinct ranges of chemical shifts: 40 to 80 ppm (tetrahedral Al, Al Td) and −22 to 22 ppm (octahedral Al, Al Oh). The shoulders that appear in the main signals at 60 ppm and 0 ppm have been assigned as tetrahedral Al in the polymorphous A and B of zeolite *BEA [
33].
Table 4 (columns 1 and 2) shows the relative distribution of Al species in the catalysts. The HB catalyst had approximately 64% Al Td in its structure. After dealumination, there was an increase in the amount of Al Td, and a decrease in the Al Oh, which was possibly a consequence of the rearrangement of Al Oh into Al Td. With the increase in the percentage of Nb over DB, there was an increase in the percentage of Al Oh. In catalytic reactions, Al Td can be characterized as a strong Brønsted acid site and Al Oh as a Lewis acid site (located outside the framework) [
4,
34]. Thus, the addition of niobium on DB zeolite promoted a decrease of Al atoms from the framework during thermal treatments, as observed by supported Nb
2O
5/ZSM-5 [
35,
36]. As a result of this, we inferred that dealumination decreased the relative amount of strong Brønsted acid sites, and increased the number of Lewis acid sites. The aqueous impregnation with Nb enriched the surface of the catalyst with an overlayer of Nb
2O
5, which can also contribute to the Lewis acid sites.
Figure 5 shows the experimental
29Si MAS NMR spectra. The deconvoluted signal spectra are displayed in
Figure S3. The relative distribution of the main signals is in
Table 4. After the modifications made to the catalysts, there was no significant shift in the Q
3 signals around −102 ppm, which refers to Si (1Al, 3Si or SiOH) in different environments and neither in Q
4 (around −111 and −115 ppm, which can be associated with the Si (0Al) environment. Zeolite *BEA presented nine different crystallographic sites [
37], which can cause different chemical shifts to the same Si (nAl) environment [
28,
34,
38]. The major modification of Q
n distribution was from HB to DB, with the decreased amount of Q
3. A possible contribution in Q
4 that should not be totally ruled out is the deposit of silica on the surface of the DB crystallite. This silica would be formed by the hydrolysis of SiF
4 that results from the decomposition of (NH
4)
2SiF
6 in the process of dealumination [
39]. After addition of Nb, the Q
n distribution was about the same of HB, which indicated that no strong modification of the zeolite structure was detected, as confirmed by FTIR and XRD measurements.
The gas phase pyridine adsorption results identified FTIR bands related to the Brønsted (1545 cm
−1) and Lewis (1447 cm
−1) sites (
Figure S4). The calorimetric titration provides the heat released after adding the probe molecule (Py) to the catalyst, which is related to the interaction between the base and the acidic sites (Brønsted and Lewis) present on the surface. The curves corresponding to the calculated enthalpy difference (ΔH) versus the number of mmoles of Py added was plotted in
Figure 6. The curves indicated that pyridine initially reacted preferentially with the stronger Brønsted acid sites (site 1), due to the most negative free energy associated with these sites [
40]. In the middle of the titration, there was a simultaneous interaction of the base with either Brønsted sites or Lewis sites. At the end of the titration, the probe molecule interacted mainly with Lewis sites and weaker hydrogen bonding sites, which may contribute to lower the average strength of site 2 [
40]. It can be observed that HB has stronger acid sites than the other catalysts, and many more sites were accessible to pyridine. Quantitatively, the strength of the sites may be divided in approximately two ranges: i) the highest enthalpies (ΔH
1) for all catalysts, which extends up to 0.15 mmoles; ii) the lower enthalpies (ΔH
2), which is from 0.15 to 0.45 mmoles of the Py added. For the HB zeolite, the range is extended up to approximately 0.70 mmoles. Clearly, it can be considered an intermediate range of enthalpies, where there is a simultaneous interaction of pyridine with either the Brønsted or Lewis sites. Thus, based on the proposed method of calculation, the different number of acid sites (n
1 and n
2) and their respective enthalpies (ΔH
1 and ΔH
2) were obtained (
Table 5).
It could be noted that the 18% Nb-DB catalyst showed a similar number (mmoles) of the two acid sites with average enthalpy—(ΔH1 + ΔH2) of ~−45 kJ mol−1) and more external area among the supported catalysts.
3.2. Ethanol Catalytic Dehydration
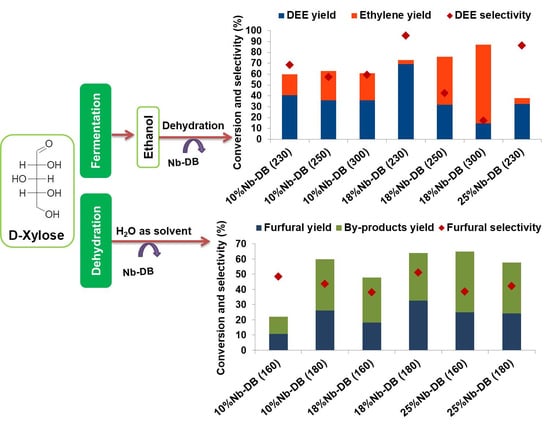
Before the dehydration reactions, three runs with ethanol (without catalyst) were carried out at the three reaction temperatures used. After that, we calculated an average of the amount of ethanol that, in fact, reached the catalyst and this amount was adopted as the total ethanol that went to the catalytic bed. From the obtained results, we carried out selectivity calculations for diethyl ether (DEE), ethylene, and other products that may have been formed. The average error in this procedure was 3%, which can be taken for the conversion and selectivity results. The percentage of the conversion of ethanol in pulses 1 and 50, at the different temperatures, are shown in
Figure S5, whereas the conversion and turnover numbers are presented in
Table 6. In a former study [
9], we confirmed that among the used catalysts, HB was the most active to the conversion of ethanol into ethylene, with percentages higher than 90%. Even at pulse 50, where it was expected to have a greater amount of coke in the catalyst, which consequently could reduce the catalytic performance, the conversions remained above 90%. The dealumination decreased the catalytic performance for DB since it presented conversions between 69% and 81%. Higher temperatures favored the conversion, and thus the reaction at 300 °C showed about 12% higher conversion than the reaction at 230 °C. Based on TON, DB was more active at 300 °C (1.7 compared to 1.5 from HB) and, generally, it was noted that TON increased with the temperature, since the conversion of ethanol also increased, and the total number of acid sites decreased (0.40 for DB compared to 0.61 for HB).
With these results, it is acceptable to infer that the Brønsted and Lewis acid sites directly influenced the catalytic dehydration. As the HB catalyst had not undergone any modification, it was the one with the most Brønsted acid sites (−ΔH1 = 105 kJ mol−1 related to the Al Td species and bridge silanol groups). Likewise, higher temperatures also contributed to the catalytic performance. The dealuminated zeolite presented fewer strong acid sites (n1 = 0.16 mmol g−1 compared to 0.21 from HB), and showed a lower catalytic conversion; however, this performance increased in the reactions at higher temperatures. The niobium addition on the support (Nb-DB) showed generally higher conversion with Nb loading, which was possibly due to the gain of the Lewis sites (−ΔH2 average values around 45 kJ mol−1). The 18%Nb-DB catalyst showed an increase in the catalytic performance (more Lewis acid sites) relative to 10%Nb-DB. Finally, the catalyst with the largest amount of supported Nb had low catalytic activity that was only compensated at 300 °C.
Much more significant than the conversion was the selectivity. In this sense, calculated TON for DEE (
Table 6 and
Figure S6) illustrates, respectively, the formation of DEE and ethylene in the first and fiftieth injection of ethanol through the catalytic bed. No other products were detected. Considering pulse 1, the selectivity for ethylene was greater than 96% with the HBEA catalyst, reached 100% when the dehydration was carried out at 300 °C, and did not produce any traces of ethylene oligomers, as observed before [
9]. The selectivity of the DB catalyst was greater than 64% at 230 °C for DEE, but declined with increasing temperature. The increase in selectivity for DEE was verified with the impregnation of niobium oxide and at lower temperature reactions, despite presenting 17% selectivity at 300 °C. The highest selectivity (95%) for DEE was obtained with the 18%Nb-DB catalyst in the reaction at 230 °C, also confirmed by TON calculation. The catalyst with the highest loading of Nb (25%Nb-DB) showed 86% selectivity at 230 °C, and only 39% at 300 °C.
From these results, it is possible to infer that the DEE production was influenced by diffusion of the reagent through the catalyst. Dealumination increased the amount of mesopores (which favors the access of reactants to the acidic sites in a bimolecular reaction), increased the hydrophobicity of the catalyst (eliminating possible interactions of water with acidic sites) [
41], and decreased the amount of Brønsted acid sites (which favor the ethylene formation)—factors that directly influence the selectivity for DEE. In addition, the low WHSV (57 h
−1) during the reaction favored the access of ethanol to the Lewis acid sites (derived from the presence of supported oxide and extra framework aluminum species). The results of these combinations made it possible to produce DEE in a highly selective manner. The percentage of carbon (coke) formed and deposited on the catalysts after the dehydration reaction (50 pulses) ranged from 4.1% for HB (230) to 0.62% for 18%Nb-DB (230). The lower coke formation of 18%Nb-DB is probably because of lower number of Brønsted acidic sites than HB, which are known to facilitate the formation of high content of carbon deposits [
1,
9,
13].
A brief comparison with other results in the literature is provided. Yang et al. [
11] used catalysts of metal organic framework with nodes of Zr
6O
8 to evaluate the dehydration of ethanol at 200 and 250 °C. Under a conversion regime lower than 10% (differential reactor), they obtained 100% selectivity for DEE, but the conversion quickly declined after 50 min. Gil-Horán et al. [
12] working at 350 °C in a isothermal fixed-bed reactor, obtained about 18% of DEE after 5 h reaction. They used modified ZSM5 (Si/Al = 80) with P and Ni as catalysts. Phung et al. [
13] studied several protonic zeolites at reaction temperatures of 180 to 200 °C. They found that H-MFI (Si/Al = 50), at a low conversion regime, had more than 70% selectivity for DEE, and only 1.4% ethylene yield. Thus, these few examples show that our results are promising for further developments.
3.3. Catalytic Conversion of Xylose
The chromatograms obtained by HPLC-RID for all catalysts applied in the reaction of xylose to furfural at temperatures of 160 and 180 °C are shown in
Figure 7. The retention time of the xylose peak was around 7.5 min, furfural was at 20.1 min and by-products peaks at 6.9 min (immediately before the xylose peak and between xylose and furfural peaks).
In the reaction without catalyst (
Figure 7), there was low conversion (intense peak due the presence of the xylose), but high selectivity for furfural, which can be considered as 100%, due to the absence/insignificance of by-product peaks in the xylose chromatogram (160 and 180 °C). The conversion improved by also increasing the temperature to the catalytic reactions, and the selectivity remained almost the same. This information corroborates that of
Figure 8, which shows that the temperature of 160 °C is still low to obtain good results from the conversion of xylose. At this temperature, despite the low conversion of xylose (~25%) and furfural production (~15%), the DB catalyst showed the highest selectivity for the desired product (~60%). The 25%Nb-DB catalyst also showed excellent results. Although the selectivity (~40%) was lower than the DB catalyst, the conversion was approximately 65%, and with a furfural production of 25%. The increase in temperature mainly influenced the conversion results of xylose.
The reaction temperature was important to the considerable change in the results of conversion and selectivity for the analysis of the reaction product without a catalyst. At 160 °C, there was no significant conversion, while at 180 °C, a higher selectivity for furfural was observed (~66%), although the conversion was only 21% and the production of furfural was 13%. Among all the catalysts applied in the reaction, the 18%Nb-DB (180 °C) catalyst was the one that showed a conversion of 64% of xylose and the highest yield of furfural (~35%).
The production of furfural w/v (%) can be analyzed for each catalyst in
Figure 9. The maximum possible percentage of furfural production in the reactant solution, considering the initial mass of the xylose (0.31 g), was around 1.59 w/v (%). According to
Figure 9, the furfural production increased with temperature and the highest production was for the 18%Nb-DB (180 °C) catalyst with ~0.5 w/v (%). This catalyst also had an increased number of Lewis acid sites.
Studies show that Brønsted acid catalysts, such as HB, convert xylose directly to furfural [
42,
43,
44]. Therefore, good results are expected for conversion and selectivity using this catalyst. In fact, there was a high conversion of xylose (~44%) from HB at 160 and 180 °C (
Figure 8). These conditions extended to selectivity (~37% and 42%, respectively) if compared to the reaction without catalyst (xylose), which only showed a considerable conversion in the reaction at 180 °C. Due to the similarity in the HB results at both temperatures, we concluded that this parameter was not the determining factor, but the Brønsted acid sites were. Despite demonstrating good conversion and selectivity results by directly transforming xylose, the high acidity of Brønsted will not only form furfural as a product, but also the selectivity of the reaction will be impaired by this factor, as can be seen in
Figure 7 and
Figure 8. The reactions carried out with HB catalyst at both temperatures (
Figure 7) indicate significant peaks (asterisks) of possible by-products.
The presence of the Lewis acid sites, predominant in the impregnated Nb catalysts, altered the path in the conversion of xylose to furfural in relation to the reaction with stronger Brønsted acid sites (
Scheme 1). Before the conversion of xylose to furfural, its isomerization to xylulose or epimerization to lyxose occurs [
45]. The dehydration of xylulose increases furfural yield when compared to xylose dehydration under similar reaction conditions [
43]. The combination of the Brønsted and Lewis acidic sites with moderate strength is essential to form enediol intermediates resulting in better conversion and selectivity [
43,
44,
45,
46]. The reaction temperature, although it did not influence the catalyst with a high number of Brønsted acid sites (HB), caused a considerable increase in the conversion for those catalysts with greater Lewis acidity. It is possible to notice in
Figure 8 that the conversion of xylose via DB catalyst increased from 25% to 35%; for 10% Nb-DB from 22% to 60% and for 18% Nb-DB from 48% to 64%, with increasing reaction temperature. Generally, the increase in the proportion of Nb
2O
5 impregnated on the catalysts increased the conversion of xylose at 160 and 180 °C. Some by-products can be the intermediates (xylulose and lixose mentioned above), due to the proximity in the retention time in relation to the peak of xylose obtained by HPLC-RID. In addition to these by-products, humins can also be generated by the reaction of furfural with xylose (Reaction 2,
Scheme 1) or by reacting with itself (Reaction 1,
Scheme 1), but it is not possible to determine them by HPLC [
42,
47].
Table 7 shows the calculated turnover number (TON) based on conversion of xylose and the formation of furfural, and the respective acidity of the catalysts (total number of acid sites, n
1 + n
2). It can be observed that TON increased with temperature and also with Nb loading. Moreover, the 18%Nb-DB catalyst showed the highest TON for yielding furfural at 180 °C. It is important to remember that these reactions were performed in water.
Figure 10 shows a comparison between the contour graphics of the furfural pattern (0.5 w/v%) and most of the products obtained through the xylose conversion reaction at 180 °C, using the 18%Nb-DB catalyst. These curves were constructed with the 3D data from the analysis by HPLC-PDA. In both graphs (a and b), the furfural retention time was around 20.1 min. In addition, the wavelength that presented the highest absorbance intensity for furfural (red region in the curves) was around 275 nm. This wavelength is commonly used to quantify furfural [
48,
49]. The information in the graph allows us to conclude that the product obtained from the reaction was actually furfural, and its concentration for the reaction at 180 °C was around 0.5 (w/v%), confirming the data in
Figure 9.
Xylose conversion reactions using solvents, such as tetrahydrofuran (THF), dimethyl sulfoxide (DMSO), toluene, butanol, and γ-valerolactone (GVL), provide higher conversion and selectivity values [
45,
46,
50,
51,
52]. Therefore, reactions carried out in a medium where only water is used as the solvent is a challenge. This type of reaction is desired from the point of view of green chemistry, as well as the need to avoid homogeneous acid catalysts (e.g., H
2SO
4, HCl).
Table 8 lists some catalysts and reaction conditions used in the literature, including two of the best results obtained in this work.
Comparing the conversion results of xylose and the selectivity to furfural with the other catalysts in
Table 8, we found that the studied catalysts obtained good results using only water as a solvent in 120 min of reaction. In addition, the time of reaction for 18%Nb-DB was half of those in the two reported studies [
50,
51]. Under slightly different reaction conditions, Vieira et al. [
45] verified that the catalyst Nb
2O
5 converted 96.8% of xylose with 42.1% selectivity for furfural. When Nb
2O
5 was dispersed on silica (30% Nb
2O
5/SiO
2), conversion decreased to 89.2%, with 37.2% selectivity for furfural. Thus, the quantity and strength of the moderate Lewis acid sites play a key role in transforming xylose into furfural.