Adsorption Kinetics of Arsenic (V) on Nanoscale Zero-Valent Iron Supported by Activated Carbon
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis and Characterization of NZVI/AC
2.2. Methods of Adsorption Kinetics
2.3. Models
2.4. The Qualitative Parameters of the Simulation and Raw Groundwater
3. Results
3.1. Adsorbent Characterization
3.2. Adsorption Kinetics of As(V) on NZVI/AC
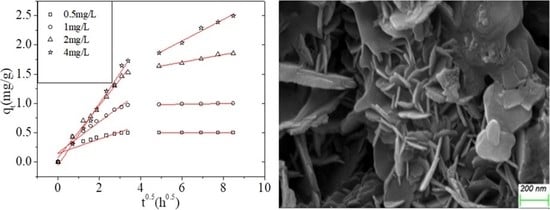
3.2.1. Effect of the Initial As(V) Concentration on Adsorption Kinetics
3.2.2. Effect of the Adsorbent Dosage on the Adsorption Kinetics
3.2.3. Effect of pH on the Adsorption Kinetics
3.2.4. Effect of Temperature on Adsorption Kinetics
3.3. As(V) Adsorption in the Presence of Other Ions
3.3.1. Effect of Single Coexisting Ions on Adsorption Kinetics
3.3.2. Multiple Coexisting Ion Effect on Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zou, Y.; Wang, X.; Khan, A. Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: A review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef] [PubMed]
- Tuček, J.; Prucek, R.; Kolařík, J. Zero-valent iron nanoparticles reduce arsenites and arsenates to As (0) firmly embedded in Core–Shell superstructure: Challenging strategy of arsenic treatment under anoxic conditions. ACS Sustain. Chem. Eng. 2017, 5, 3027–3038. [Google Scholar] [CrossRef]
- Xu, J.; Cao, Z.; Zhang, Y. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism. Chemosphere 2018, 195, 351–364. [Google Scholar] [CrossRef]
- Maiti, A.; Mishra, S.; Mohit Chaudhary, M. Nanoscale materials for arsenic removal from water. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 707–733. [Google Scholar]
- Shang, J.; Zong, M.; Yu, Y. Removal of chromium (VI) from water using nanoscale zerovalent iron particles supported on herb-residue biochar. J. Environ. Manag. 2017, 197, 331–337. [Google Scholar] [CrossRef]
- Shi, L.; Zhou, Y.; Chen, Z. Simultaneous adsorption and degradation of Zn2+ and Cu2+ from wastewaters using nanoscale zero-valent iron impregnated with clays. Environ. Sci. Pollut. Res. 2013, 20, 3639–3648. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, C.; Choi, I. Arsenic removal using mesoporous alumina prepared via a templating method. Environ. Sci. Technol. 2004, 38, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Penke, Y.K.; Anantharaman, G.; Ramkumar, J.; Kar, K.K. Redox synergistic Mn-Al-Fe and Cu-Al-Fe ternary metal oxide nano adsorbents for arsenic remediation with environmentally stable As(0) formation. J. Hazard. Mater. 2019, 364, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Peng, S.; Yan, K. Enhanced As (III) sequestration using sulfide-modified nano-scale zero-valent iron with a characteristic core–shell structure: Sulfidation and as distribution. ACS Sustain. Chem. Eng. 2018, 6, 3039–3048. [Google Scholar] [CrossRef]
- Jain, A.; Agarwal, M. Synthesising zero valent iron supported on alumina for removal of arsenic from drinking water. Interdiscip. Environ. Rev. 2017, 18, 108–123. [Google Scholar] [CrossRef]
- Tan, G.; Mao, Y.; Wang, H. Comparison of biochar- and activated carbon-supported zerovalent iron for the removal of Se(IV) and Se(VI): Influence of pH, ionic strength, and natural organic matter. Environ. Sci. Pollut. Res. 2019, 26, 21609–21618. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Y.; Wu, X. Removal of arsenic from water by supported nano zero-valent iron on activated carbon. J. Hazard. Mater. 2009, 172, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Demopoulos, G.P. Adsorption of arsenate onto ferrihydrite from aqueous solution: Influence of media (sulfate vs. nitrate), added gypsum, and pH alteration. Environ. Sci. Technol. 2005, 39, 9523–9527. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, B.; Li, Y. Adsorptive removal of arsenate from aqueous solutions by biochar supported zero-valent iron nanocomposite: Batch and continuous flow tests. J. Hazard. Mater. 2017, 322, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Lv, L.; Pan, B.C. Critical review in adsorption kinetic models. J. Zhejiang Univ. Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Kinetics of adsorption of metal ions on inorganic materials: A review. Adv. Colloid Interface Sci. 2011, 162, 39–58. [Google Scholar] [CrossRef]
- Lackovic, J.A.; Nikolaidis, N.P.; Dobbs, G.M. Inorganic arsenic removal by zero-valent iron. Environ. Eng. Sci. 2000, 17, 29–39. [Google Scholar] [CrossRef]
- Kanel, S.R.; Grenèche, J.M.; Choi, H. Arsenic(V) removal from groundwater using nano scale zero-valent iron as a colloidal reactive barrier material. Environ. Sci. Technol. 2006, 40, 2045–2050. [Google Scholar] [CrossRef]
- Juang, R.S.; Tseng, R.L.; Wu, F.C. Adsorption behavior of reactive dyes from aqueous solutions on chitosan. J. Chem. Technol. Biotechnol. Int. Res. Process. Clean. Technol. 1997, 70, 391–399. [Google Scholar] [CrossRef]
- Oke, I.A.; Olarinoye, N.O.; Adewusi, S.R.A. Adsorption kinetics for arsenic removal from aqueous solutions by untreated powdered eggshell. Adsorption 2008, 14, 73–83. [Google Scholar] [CrossRef]
- Doke, K.M.; Khan, E.M. Equilibrium, kinetic and diffusion mechanism of Cr(VI) adsorption onto activated carbon derived from wood apple shell. Arab. J. Chem. 2017, 10, S252–S260. [Google Scholar] [CrossRef] [Green Version]
- Hameed, B.H.; Ahmad, A.A.; Aziz, N. Isotherms, kinetics and thermodynamics of acid dye adsorption on activated palm ash. Chem. Eng. J. 2007, 133, 195–203. [Google Scholar] [CrossRef]
- Ong, S.A.; Seng, C.E.; Lim, P. Kinetics of adsorption of Cu (II) and Cd (II) from aqueous solution on rice husk and modified rice husk. Electron. J. Environ. Agric. Food Chem. 2007, 6, 1764–1774. [Google Scholar]
- Raven, K.P.; Jain, A.; Loeppert, R.H. Arsenite and arsenate adsorption on ferrihydrite: Kinetics, equilibrium, and adsorption envelopes. Environ. Sci. Technol. 1998, 32, 344–349. [Google Scholar] [CrossRef]
- Guo, X.; Chen, F. Removal of arsenic by bead cellulose loaded with iron oxyhydroxide from groundwater. Environ. Sci. Technol. 2005, 39, 6808–6818. [Google Scholar] [CrossRef]
- Hamadi, N.K.; Chen, X.D.; Farid, M.M. Adsorption kinetics for the removal of chromium(VI) from aqueous solution by adsorbents derived from used tyres and sawdust. Chem. Eng. J. 2001, 84, 95–105. [Google Scholar] [CrossRef]
- Kanel, S.R.; Manning, B.; Charlet, L. Removal of arsenic (III) from groundwater by nanoscale zero-valent iron. Environ. Sci. Technol. 2005, 39, 1291–1298. [Google Scholar] [CrossRef]
- Su, C.; Puls, R.W. Arsenate and arsenite removal by zerovalent iron: Effects of phosphate, silicate, carbonate, borate, sulfate, chromate, molybdate, and nitrate, relative to chloride. Environ. Sci. Technol. 2001, 35, 4562–4568. [Google Scholar] [CrossRef]
- Taty-Costodes, V.C.; Fauduet, H.; Porte, C. Removal of Cd (II) and Pb (II) ions, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris. J. Hazard. Mater. 2003, 105, 121–142. [Google Scholar] [CrossRef]
- Ali, R.M.; Hamad, H.A.; Hussein, M.M. Potential of using green adsorbent of heavy metal removal from aqueous solutions: Adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol. Eng. 2016, 91, 317–332. [Google Scholar] [CrossRef]
- Georgopoulou, A.N.; Adam, R.; Raptopoulou, C.P. Isomorphous replacement of M(II) ions in M(II)-Gd(III) dimers (M(II) = Cu(II), Mn(II), Ni(II), Co(II), Zn(II)): Magnetic studies of the products. Dalton Trans. 2010, 39, 5020–5027. [Google Scholar] [CrossRef] [PubMed]








| As (mg/L) | pH | TOC (mg/L) | NH3-N (mg/L) | Fe (mg/L) | SO42− (mg/L) | Cl− (mg/L) | F− (mg/L) | Na+ (mg/L) | K+ (mg/L) | Al3+ (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1.971 | 7.58 | 12.3 | 0.475 | 4.6 | 349 | 174 | 1.62 | 137 | 3.9 | 1.04 |
| Parameter | Weber–Morris Diffusion | |||
|---|---|---|---|---|
| 1st Step | 2nd Step | |||
| C0 (mg/L) | kid1 | R2 | kid2 | R2 |
| 0.5 | 0.1224 | 0.7636 | 0.0004 | 0.9774 |
| 1.0 | 0.2632 | 0.9293 | 0.0060 | 0.9341 |
| 2.0 | 0.4449 | 0.9900 | 0.0648 | 0.9673 |
| 4.0 | 0.5251 | 0.9913 | 0.1835 | 0.9769 |
| Parameter | Weber–Morris Diffusion | |||
|---|---|---|---|---|
| 1st Step | 2nd Step | |||
| C0 (mg/L) | kid1 | R2 | kid2 | R2 |
| 0.5 | 0.5515 | 0.9838 | 0.1677 | 0.9399 |
| 1.0 | 0.4449 | 0.9900 | 0.0647 | 0.9672 |
| 1.5 | 0.3203 | 0.9662 | 0.0326 | 0.9934 |
| 2.0 | 0.2482 | 0.7790 | 0.0012 | 0.9234 |
| Parameter | Weber–Morris Diffusion | |||
|---|---|---|---|---|
| 1st Step | 2nd Step | |||
| pH | kid1 | R2 | kid2 | R2 |
| 3.5 | 0.4921 | 0.9362 | 0.0355 | 0.9731 |
| 6.5 | 0.4449 | 0.9899 | 0.0647 | 0.9673 |
| 9.5 | 0.1654 | 0.9532 | 0.0409 | 0.9646 |
| Parameter | Weber–Morris Diffusion | |||
|---|---|---|---|---|
| 1st Step | 2nd Step | |||
| T(K) | kid1 | R2 | kid2 | R2 |
| 298 | 0.4449 | 0.9900 | 0.0647 | 0.9673 |
| 308 | 0.4657 | 0.9677 | 0.0203 | 0.9926 |
| 318 | 0.4664 | 0.9503 | 0.0283 | 0.8860 |
| Parameter | Weber–Morris Diffusion | |||
|---|---|---|---|---|
| 1st Step | 2nd Step | |||
| Single Coexisting Ions | kid1 | R2 | kid2 | R2 |
| CK 1 | 0.4449 | 0.9900 | 0.0647 | 0.9673 |
| 0.2633 | 0.9577 | 0.0776 | 0.8741 | |
| 0.2123 | 0.9756 | 0.0638 | 0.9180 | |
| Parameter | Weber–Morris Diffusion | |||
|---|---|---|---|---|
| 1st Step | 2nd Step | |||
| Multiple Coexisting Ions | kid1 | R2 | kid2 | R2 |
| CK | 0.4449 | 0.9900 | 0.0648 | 0.9673 |
| multiple coexisting ions | 0.1447 | 0.9679 | 0.0602 | 0.9722 |
| Parameter | Weber–Morris Diffusion | |||
|---|---|---|---|---|
| 1st Step | 2nd Step | |||
| kid1 | R2 | kid2 | R2 | |
| simulation water | 0.09725 | 0.9922 | 0.01922 | 0.9907 |
| raw groundwater | 0.0832 | 0.9902 | 0.02323 | 0.9952 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Shi, M.; Zhang, X.; Liu, B.; Yao, D. Adsorption Kinetics of Arsenic (V) on Nanoscale Zero-Valent Iron Supported by Activated Carbon. Nanomaterials 2020, 10, 1791. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10091791
Zhu H, Shi M, Zhang X, Liu B, Yao D. Adsorption Kinetics of Arsenic (V) on Nanoscale Zero-Valent Iron Supported by Activated Carbon. Nanomaterials. 2020; 10(9):1791. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10091791
Chicago/Turabian StyleZhu, Huijie, Mingyan Shi, Xiuji Zhang, Bo Liu, and Dahu Yao. 2020. "Adsorption Kinetics of Arsenic (V) on Nanoscale Zero-Valent Iron Supported by Activated Carbon" Nanomaterials 10, no. 9: 1791. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10091791