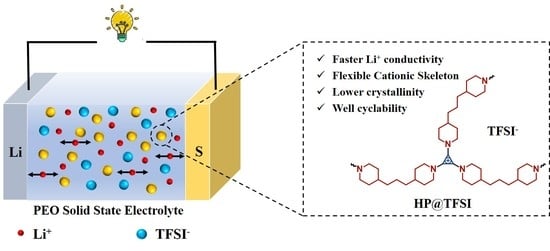
Cationic Cyclopropenium-Based Hyper-Crosslinked Polymer Enhanced Polyethylene Oxide Composite Electrolyte for All-Solid-State Li-S Battery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Fabrication of HP@Cl and HP@TFSI
2.3. Preparation of Sulfur Composite and Electrode
2.4. Batteries Assembling Steps
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qin, K.; Holguin, K.; Mohammadiroudbari, M.; Huang, J.H.; Kim, E.Y.S.; Hall, R.; Luo, C. Strategies in Structure and Electrolyte Design for High-Performance Lithium Metal Batteries. Adv. Funct. Mater. 2021, 31, 2009694. [Google Scholar] [CrossRef]
- Umeshbabu, E.; Zheng, B.; Yang, Y. Recent Progress in All-Solid-State Lithium-Sulfur Batteries using high Li-ion conductive solid electrolytes. Electrochem. Energ. Rev. 2019, 2, 199–230. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.W.; Wang, S.F. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, X.; Adair, K.; Zhang, H.; Sun, X. Structural Design of Lithium-Sulfur Batteries: From Fundamental Research to Practical Application. Electrochem. Energy Rev. 2018, 1, 239–293. [Google Scholar] [CrossRef] [Green Version]
- Manthiram, A.; Fu, Y.; Chung, S.H.; Zu, C.; Su, Y.S. Rechargeable Lithium−Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Advances in lithium–sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 2016, 1, 16132. [Google Scholar] [CrossRef]
- Evers, S.; Nazar, L.F. New Approaches for High Energy Density Lithium-Sulfur Battery Cathodes. Acc. Chem. Res. 2013, 46, 1135–1143. [Google Scholar] [CrossRef]
- Zhao, Q.; Zheng, J.X.; Archer, L. Interphases in Lithium−Sulfur Batteries: Toward Deployable Devices with Competitive Energy Density and Stability. ACS Energy Lett. 2018, 3, 2104–2133. [Google Scholar] [CrossRef]
- Urbonaite, S.; Poux, T.; Novák, P. Progress towards Commercially Viable Li-S Battery Cells. Adv. Energy Mater. 2015, 5, 1500118. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Liaw, B.Y.; Metzler, V.; Zhang, J.B. A review of lithium deposition in lithium-ion and lithium metal secondary batteries. J. Power Sources 2014, 254, 168–182. [Google Scholar] [CrossRef]
- Gadjourova, Z.; Andreev, Y.G.; Tunstall, D.P.; Bruce, P.G. Ionic conductivity in crystalline polymer electrolytes. Nature 2011, 412, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Judez, X.; Zhang, H.; Li, C.; González-Marcos, J.A.; Zhou, Z.; Armand, M.; Rodriguez-Martinez, L.M. Lithium Bis (Fluorosulfonyl) imide/Poly (ethylene oxide) Polymer Electrolyte for All Solid-State Li-S Cell. J. Phys. Chem. Lett. 2017, 8, 1956–1960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, C.Y.; Zheng, L.P.; Xu, F.; Feng, W.F.; Li, H.; Huang, X.J.; Armand, M.; Nie, J.; Zhou, Z.B. Lithium bis (fluorosulfonyl) imide/poly (ethylene oxide) polymer electrolyte. Electrochim. Acta 2014, 133, 529–538. [Google Scholar] [CrossRef]
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Beyond PEO-Alternative host materials for Li+-conducting solid polymer electrolytes. Prog. Polym. Sci. 2018, 81, 114–143. [Google Scholar] [CrossRef]
- Pitawala, H.M.J.C.; Dissanayake, M.A.K.L.; Seneviratne, V.A. Combined effect of Al2O3 nano-fillers and EC plasticizer on ionic conductivity enhancement in the solid polymer electrolyte (PEO)9LiTf. Solid State Ion. 2007, 178, 885–888. [Google Scholar] [CrossRef]
- Maitra, P.; Ding, J.; Huang, H.; Wunder, S.L. Poly (ethylene oxide) Silananted Nanosize Fumed Silica: DSC and TGA Characterization of the Surface. Langmuir 2003, 19, 8994–9004. [Google Scholar] [CrossRef]
- Reddy, M.J.; Chu, P.P.; Kumar, J.S.; Rao, U.V.S. Inhibited crystallization and its effect on conductivity in a nano-sized Fe oxide composite PEO solid electrolyte. J. Power Sources 2016, 161, 535–540. [Google Scholar] [CrossRef]
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem. Soc. Rev. 2014, 43, 4714–4727. [Google Scholar] [CrossRef]
- Bron, P.; Johansson, S.; Zick, K.; Gunne, J.S.A.D.; Dehnen, S.; Roling, B. Li10SnP2S12: An Affordable Lithium Superionic Conductor. J. Am. Chem. Soc. 2013, 135, 15694–15697. [Google Scholar] [CrossRef]
- Kato, Y.; Kawamoto, K.; Kanno, R.; Hirayama, M. Discharge Performance of All-Solid-State Battery Using a Lithium Superionic Conductor Li10GeP2S12. Electrochemistry 2012, 80, 749–751. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.Q.; Ding, H.M.; Yuan, D.Q.; Wang, B.S.; Wang, C. A Pyrene-Based, Fluorescent Three-Dimensional Covalent Organic Framework. J. Am. Chem. Soc. 2016, 138, 3302–3305. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.Y.; Xu, G.Y.; Barnes, M.; Li, Y.L.; Tseng, C.-P.; Zhang, Z.Q.; Zhang, J.-J.; Zhu, Y.F.; Khalil, S.; Rahman, M.M.; et al. Covalent Organic Frameworks for Batteries. Adv. Funct. Mater. 2021, 31, 2100505. [Google Scholar] [CrossRef]
- Cao, S.; Li, B.; Zhu, R.M.; Pang, H. Design and synthesis of covalent organic frameworks towards energy and environment fields. Chem. Eng. J. 2019, 355, 602–623. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Y.; Dong, Y.; Li, J.; Li, S.; Shao, P.; Feng, X.; Wang, B. Fast Ion Transport Pathway Provided by Polyethylene Glycol Confined in Covalent Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 1923–1927. [Google Scholar] [CrossRef]
- Chen, H.; Tu, H.; Hu, C.; Liu, Y.; Dong, D.; Sun, Y.; Dai, Y.; Wang, S.; Qian, H.; Lin, Z.; et al. Cationic Covalent Organic Framework Nanosheets for Fast Li-ion Conduction. J. Am. Chem. Soc. 2018, 140, 896–899. [Google Scholar] [CrossRef]
- Jiang, Y.; Freyer, J.L.; Cotanda, P.; Brucks, S.D.; Killops, K.L.; Bandar, J.S.; Torsitano, C.; Balsara, N.P.; Lambert, T.H.; Campos, L.M. The evolution of cyclopropenium ions into functional polyelectrolytes. Nat. Commun. 2015, 6, 5950. [Google Scholar] [CrossRef] [Green Version]
- Mir, R.; Dudding, T. Bis(amino)cyclopropenium Trifluor-oborates: Synthesis, Hydrolytic Stability Studies, and DFT Insights. J. Org. Chem. 2018, 83, 4384–4388. [Google Scholar] [CrossRef]
- Montoto, E.C.; Cao, Y.; Hernández-Burgos, K.; Sevov, C.S.; Braten, M.N.; Helms, B.A.; Moore, J.S.; Rodríguez-López, J. Effect of the Backbone Tether on the Electrochemical Properties of Soluble Cyclopropenium Redox-active Polymers. Macromolecules 2018, 51, 3539–3546. [Google Scholar] [CrossRef]
- Steinman, N.Y.; Starr, R.L.; Brucks, S.D.; Belay, C.; Meir, R.; Golenser, J.; Campos, L.M.; Domb, A.J. Cyclopropenium-Based Biodegradable Polymers. Macromolecules 2019, 52, 3543–3550. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, H.F.; Shi, J.J.; Li, X.N.; Zhang, X.J.; Qu, X.W. Cyclopropenium Cationic-Based Covalent Organic Polymer Enhanced Polyethylene Oxide Composite Polymer Electrolyte for All-Solid-State Li-S Battery. ACS Appl. Mater. Interfaces 2021, 13, 16469–16477. [Google Scholar] [CrossRef]
- Li, X.; Wang, D.; Wang, H.; Yan, H.; Gong, Z.; Yang, Y. Poly(ethylene oxide)-Li10SnP2S12 Composite Polymer Electrolyte Enables High-Performance All-Solid-State Lithium Sulfur Battery. ACS Appl. Mater. Interfaces 2019, 11, 22745–22753. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.-W.; Mu, Z.-J.; Cao, C.; Li, Z.; Wang, T.-X.; Li, Y.; Ding, X.; Han, B.-H.; Feng, W. Cationic Covalent Organic Framework Based All-Solid-State Electrolytes. Mater. Chem. Front. 2020, 4, 1164–1173. [Google Scholar] [CrossRef]
- Minamitani, E.; Nakanishi, H.; Agerico Dino, W.; Kasai, H. Spectroscopic Profiles of a Magnetic Dimer on a Metal Surface. Solid State Commun. 2009, 149, 1241–1243. [Google Scholar] [CrossRef]
- Horike, S.; Umeyama, D.; Kitagawa, S. Ion Conductivity and Transport by Porous Coordination Polymers and Metal-organic Frameworks. Acc. Chem. Res. 2013, 46, 2376–2384. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, Y.; Li, W.; Meng, L.; Bai, Y.; Chen, G. Organic-Inorganic Multi-Scale Enhanced Interfacial Engineering of Solid Sulfide Electrolyte in Li-S Battery. Chem. Eng. J. 2020, 396, 125334. [Google Scholar] [CrossRef]





| Electrolytes | Tm/°C | ΔHm/J·g−1 | |
|---|---|---|---|
| PEO-LiTFSI | 57.5 | 60.25 | 40.6 |
| PEO-5%HP@TFSI | 57.2 | 56.82 | 40.2 |
| PEO-10%HP@TFSI | 56.9 | 55.15 | 38.6 |
| PEO-20%HP@TFSI | 56.3 | 45.3 | 36.6 |
| Electrolyte | I0/mA | Is/mA | R0/Ω | Rs/Ω | ΔV/mV | |
|---|---|---|---|---|---|---|
| PEO/LiTFSI | 0.036 | 0.021 | 236.4 | 248.1 | 10 | 0.183 |
| PEO-5%HP@TFSI | 0.036 | 0.020 | 146.2 | 159.4 | 10 | 0.287 |
| PEO-10%HP@TFSI | 0.035 | 0.015 | 135.3 | 168.7 | 10 | 0.319 |
| PEO-20%HP@TFSI | 0.042 | 0.024 | 77.6 | 108.1 | 10 | 0.521 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, S.; Wang, Y.; Ji, H.; Zhang, X.; Shi, J.; Feng, Y.; Qu, X. Cationic Cyclopropenium-Based Hyper-Crosslinked Polymer Enhanced Polyethylene Oxide Composite Electrolyte for All-Solid-State Li-S Battery. Nanomaterials 2021, 11, 2562. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11102562
Lian S, Wang Y, Ji H, Zhang X, Shi J, Feng Y, Qu X. Cationic Cyclopropenium-Based Hyper-Crosslinked Polymer Enhanced Polyethylene Oxide Composite Electrolyte for All-Solid-State Li-S Battery. Nanomaterials. 2021; 11(10):2562. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11102562
Chicago/Turabian StyleLian, Shuang, Yu Wang, Haifeng Ji, Xiaojie Zhang, Jingjing Shi, Yi Feng, and Xiongwei Qu. 2021. "Cationic Cyclopropenium-Based Hyper-Crosslinked Polymer Enhanced Polyethylene Oxide Composite Electrolyte for All-Solid-State Li-S Battery" Nanomaterials 11, no. 10: 2562. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11102562