3.1. Chemical Stability Diagram
The formation of the most probable iron-related defects in HAp depends most notably on the chemical potentials of the several elements involved. Each chemical potential can vary within a certain range, limited by thermodynamic stability conditions of the HAp crystal itself. We estimate the ranges using Equation (
2) with respect to a set of boundary phases
. The methodology used to find the structure and energy of each phase was identical to that used for the HAp supercell. This includes the exchange-correlation functional and energy cutoffs. The first candidates for the bordering phases are those involved in the HAp synthesis. There is a variety of production routes, but we will only consider reactants whose species are common to those found in HAp, namely CaO [
6], Ca(OH)
[
8], H
PO
, H
O and P
O
[
18]. Next, we considered calcium phosphates: dicalcium phosphate dihydrate CaHPO
H
O = DCPD (mineral brushite), anhydrous dicalcium phosphate CaHPO
= DCPA (mineral monetite), tricalcium phosphate Ca
(PO
= TCP and tetracalcium phosphate Ca
(PO
)
O = TTCP. The last two have [Ca]: [P] concentration ratio of 1.5 and 2.0, respectively, while HAp has an intermediate [Ca]: [P] ratio of
.
Table 1 presents the formation energies
, calculated according to Equation (
3) within HSE, for the materials enumerated above. The overall agreement with the reference data [
53,
54] (including the energy, cell volume and bulk modulus) is in line with the usual accuracy of hybrid-DFT. The phosphoric acid and water were calculated in a gas phase (single molecules in large periodic box), hence the lack of cell volume and bulk modulus.
From the data of
Table 1 and Equation (
2), we can estimate the chemical potential ranges within which HAp is thermodynamically stable. We can also reduce the number of independent chemical potentials, for instance to
,
and
, by expressing the chemical potential of oxygen as a function of
of the remaining elements (c.f. Equation (
2)),
Let us look first at the range of hydrogen chemical potentials. In this case, phases with stoichiometry (not) satisfying give lower (upper) bounds for . In particular, the planes corresponding to CaO, TCP and pure O may form lower bounds, while planes for hydrogen-containing phases may be responsible for upper bounds.
According to our calculations, the HAp stability domain is limited by CaO, Ca(OH
, P, H
, DCPD and DCPA phase planes.
Figure 2 illustrates the domain of chemical stability of HAp as a convex faceted hull with vertices
(with
). These correspond to regions in phase space where three different phases coexists with HAp.
Table S1 in Supplementary Materials contains the calculated values of chemical potentials at these intersection points. We note that there is no boundary with Ca metal, with the upper limit for
being
eV. This means that if
was assumed, formation energies of defects involving Ca substitutions would be affected by an error of
eV. In the analysis regarding substitutional iron defects, we will consider the following chemical phase space conditions:
Ca-rich and P-rich, points and , eV, ;
Ca-poor, point , eV, eV;
P-poor, points and , eV, eV.
3.3. Neutral Iron Defects
We start by considering neutral iron defects based on structures previously proposed in the literature.
Table 3 summarizes the relevant data collected. The first seven rows correspond to the Fe
substitutions, while the next five to iron interstitials. There is no apparent correlation between the defect structure and sample preparation conditions (e.g., Fe concentration or synthesis temperature).
We considered Ca(I), Ca(II), as well as P substitutions, the later being unexplored in the literature.
Figure 3a–c illustrate the substitutional defect structures obtained after relaxation, while
Figure 3d–g depict the interstitial structures. The relaxation of the structure where iron was initially set up with the Fe
configuration (Wyckoff position
), resulted in another structure, where iron is displaced away from the center of the channel. This new structure is denoted as Fe
(
Figure 3f) and approximately corresponds to Wyckoff position
. In order to achieve a stable Fe
configuration, one of the nearest OH groups has to be flipped (
Figure 3d). Another interstitial position with the iron ion displaced from the center of the channel is labeled as Fe
(
Figure 3e) and corresponds to Wyckoff position
. The Fe
structure may be slightly modified by flipping of the neighboring OH group, leading to a minute (
eV) benefit in the total energy. Such small difference is lower than the flipping of isolated OH, estimated as 0.22 eV. Our result suggest that OH flipping may be stimulated by the presence of interstitial iron in the channel.
Finally, we considered iron inserted in the region between PO
groups (Wyckoff position
), marked as Fe
(
Figure 3g). At this location, the negatively charged PO
groups are expected to screen the positive charge of the iron ion. Although we scanned the relative stability of other structures, those whose formation energy was above 7 eV were discarded and not investigated further (e.g., Fe
and Fe
in the vicinity of the Ca(I) column).
Table 4 shows the formation energies of the most stable defects, estimated according to Equation (
1) using chemical potentials at extreme points of the HAp stability diagram, namely for material grown under Ca- and P-rich (
and
), Ca-poor (
) and P-poor (
) conditions. At Ca- and P-rich conditions, the
defect is likely to be the most probable as it shows the lowest formation energy. Other stable defects (within less that 1 eV above the ground state) are
and
substitutions. Other interstitial sites (
,
) have higher formation energies. However, they still should be considered since hole or electron trapping may stabilize them.
At Ca-poor conditions
defects are the most stable, with formation energy
5 eV below that of
. A low value of
at Ca-poor conditions (
in
Figure 2) leads to an easier depletion of phosphorus and to the stabilization of
as well. Subsequently, at P-poor conditions this effect is further enhanced and
becomes nearly 4 eV more stable than
, and about 7.5 eV than the most favorable interstitial defect,
.
Table 4 reports the number of oxygen atoms neighboring iron (coordination number), their respective Fe-O distances, and the magnetic moment of each defect. The six-fold coordination of Fe
(
Figure 3a) is in line with the results of Jiang et al. [
15], Low et al. [
16] and Zilm et al. [
17]. The five-fold coordinated Fe
structure differs: six-fold coordinated iron is proposed by Jiang et al, while four-fold – by Zilm et al. However, we may count six neighbors if we include the far oxygen neighbor at distance
Å marked with dashed line on
Figure 3b. Zilm et al obtained two oxygen atoms at high distances of
and
Å, but excluded them from the neighbors count, resulting in four-fold coordination.
The structure of Fe
with two O(IV) neighbors shows Fe-O distances
Å longer than those observed by XRD [
18]. This may result from the XRD analysis, which gives an ordered structure where oxygen atoms are fixed to crystallographic sites, or may reflect the distances of a positively charged state, where the iron cation is closer to the oxygen anions. Fe
configurations have four-fold coordinated iron. This is at variance with the XRD study of Gomes et al. [
18], where three-fold coordinated iron was found, but is in line with XRD results of Kato et al. [
19]. The only three-fold coordination of Fe that we find is in the Fe
structure (
Figure 4e). However, the geometry differs from that proposed in Ref. [
18], where Fe connects to one oxygen atom from a neighboring PO
group and two O atoms from OH groups. In general, the calculated Fe-O distances look slightly overestimated. Of course, the picture could improve when considering charged cells.
3.4. Charged Iron Defects
Iron (Fe:4s3d) is not isoelectronic with respect to the species being replaced (Ca:4s and P:3s3p). Hence, Fe impurities are expected to create states within the band gap of HAp. These states are localized and may act as hole or electron traps. The description of the Fe-HAp requires the consideration these cases, which can be accounted for by changing the occupation of the highest occupied gap states of the defective supercell. In a real crystal, the capture of a hole (creation of a local positive charge) can be compensated by numerous possibilities: cation vacancies , , foreign anion interstitials like [CO], etc., thus leading to a lowering of the Fermi energy (electron chemical potential), We leave the exact mechanisms of charge compensation out of the scope of this work.
An qualitative picture of the charge states allowed for each defect can be found from the respective Kohn-Sham levels in the band gap [
61]. Positive charge states (hole trapping) requires the presence of filled states in the gap, while the negatively charged defects (electron trapping) requires the presence of empty states.
Figure 4 illustrates the energy of the one-electron states in the band gap obtained from spin-polarized calculations of substitutional and interstitial defects in the neutral charge state.
Substitutional iron on Ca sites, Fe, have six electrons in the 3d shell, but after the first ionization all filled states move below the valence band top. Hence, further ionization would require an energy equivalent to the band gap width (∼7 eV), which essentially tell us that Fe defects can be single donors, but not double donors. Unoccupied states of Fe are rather close to the conduction band, suggesting that they are not acceptors either (can not trap electrons).
Iron on the phosphorous site in the Fe state (neutral charge state) has three filled one-electron states and 7 empty states in the gap (3d configuration). We will show below that Fe can trap one hole or up to two electrons, becoming Fe or Fe and Fe states, respectively.
Neutral interstitial defects show only filled states in the gap. These can trap up to three holes, thus leading to Fe, Fe and Fe, respectively.
We found that the structure of the defects depend strongly on the local charge.
Figure 5 illustrates some of the most remarkable structural changes of the defects induced by the capture of electrons and holes. Additional reconfigurations are depicted in
Figure S1 in Supplementary Information.
Table 5 presents the number of nearest oxygen neighbors to the Fe ion (
), the range of Fe-O bond lengths (
), as well as the magnetic moment of the defect (
). Note, that some states exhibit a high magnetization, up to
for Fe
and Fe
. These are to be compared to the case of neutral defects, where the magnetic moment was at most
.
In general, an increase of the positive charge leads to a decrease of Fe-O bond lengths. This effect is strikingly illustrated in
Figure 5h,i, which showcase the Fe
–Fe
sequence of defects. This rule is understandably violated when there is a change in the coordination of the Fe ion, and therefore, a significant modification of the local electrostatics. Examples are the increases of
in
,
, or
upon hole capture,
(
Figure 5f,g),
The above processes are reversible, i.e., electron trapping at (or hole emission from) the positively charged defects result in the recovery of the longer bond lengths. As expected, HAp cations repel the iron ion. In the case of
without OH flipping, this effect leads to migration of a proton from OH to a close PO
group (see
Figure 5d,e). We found that the high-symmetry defect configuration
is not stable and spontaneously transforms to
. Hence, upon hole capture by (or electron emission from)
, the iron moves toward the edge of the OH channel (see
Figure 5a,b),
The reverse process involves overcoming a barrier (not calculated), and
is not recovered spontaneously (see
Figure 5b,c).
The formation energy of a charged defect is a function of the Fermi energy (c.f. Equation (
1)). This dependence is clearly illustrated in
Figure 6a,b, which show the results for Ca- and P-rich material and for Ca-poor HAp, respectively. The red shadow area on both diagrams indicates the whole range for the formation energy of Fe
. The upper bound corresponds to P-rich conditions, while the lower bound to P-poor material. The solid red line in
Figure 6b shows the formation energy of Fe
under Ca-poor conditions. Thick dashed-dotted lines correspond to formation energies of Fe
defects, and thick solid line and other non-solid lines correspond to interstitial defects (see legend).
According to our results, the phosphorous substitutions, especially Fe (charge state ) is a rather stable species when there is abundance of electrons in the material (n-type HAp). This is even more evidenced in Ca-poor and P-poor conditions, where in p-type material we expect Fe, Fe and even Fe to become more stable and compete with other species, namely Fe and Fe.
However, the phosphorous substitutions were not previously considered in the literature, since the replacing of phosphorus by iron cation requires the breaking of pretty stable P–O bonds of PO
group. Alternatively, the replacement of whole PO
group by FeO
one will provide the same structural result. The obtained most probable Fe
configuration is tetrahedral coordinated ferric cation ([FeO
group) is not unusual and can be found in magnetite [
52] or in iron-phosphate glass [
62].
Regarding substitutional Fe at calcium sites, we find that Fe
is the most probable form under Ca-poor and p-type conditions (
Figure 6b). We note that despite Fe
having lower formation energy than Fe
in n-type HAp, the Fe
species is even more stable, thus making the phosphorus substitution more probable than a replacement of calcium.
In contrast, under Ca- and P-rich conditions (
Figure 6a) the interstitial defects are expected to prevail, especially in p-type and intrinsic HAp. Depending on the Fermi level location, the most probable states are Fe
, Fe
and Fe
defects. The two-fold coordinated Fe
defect, is more favorable in n-type HAp, yet again, Fe
is more stable and more likely to form.
We can compare the obtained local atomic structure of iron defects in HAp (see
Table 4 and
Table 5) with those reported in the literature (
Table 3). Iron substitutions with long distances
Å and 6 oxygen neighbors [
15,
16] are best described by Fe
. The slightly more favorable Fe
substitution has 5 oxygen neighbors, which could explain the result of Boda et al. [
6]. The iron with only two neighbors in the study of Gomes et al. [
18] could be described by
, although the small Fe-O distances of 1.7 Å are only reproduced by Fe
, which is rather unstable. Iron with three [
18] or four [
19] oxygen neighbors at distances
Å can be accounted for by
or Fe
defects.
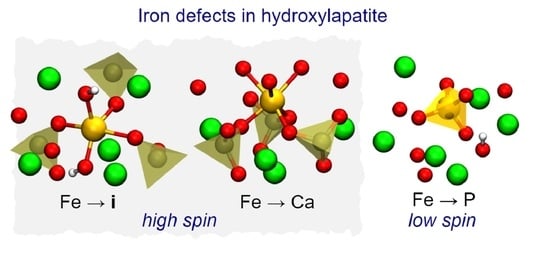
In summary, we find that Fe-HAp can contain both substitutional and interstitial defects depending on the preparation conditions. The phosphorus substitutions have iron in low-spin states (,), making them less useful for many applications envisaged for Fe-HAp. To avoid those defects the synthesis should be performed closer to P-rich and p-type conditions. In that case we expect the formation of high-spin defects with (Fe and Fe) and (Fe).
3.5. Fe K-XANES of Fe-HAp
The near-edge structure of X-ray absorption (XANES) spectra is particularly sensitive to the details of local atomic structure of the absorbing element [
51]. X-ray absorption spectroscopy has been applied to materials without long range order, and that includes the Fe-HAp. We consider the experimental spectra of Fe-HAp published by Gomes et al. [
18]. We also keep the notation of the original study regarding the experimental conditions, i.e., 15Fe-500 and 15Fe-1100 which correspond to 15 mol % of Fe per HAp unit cell of samples sintered at 500 °C and 1100 °C, respectively.
The top three (black colored) curves in
Figure 7 shows the experimental data of Gomes et al. [
18] for Fe-HAp and for magnetite (Fe
O
). The latter was used as a reference for the alignment of the theoretical energy scale to the experimental one. Greek letters mark the main spectral features: pre-edge (
), the main peak (
), its satellite peaks (
and
), and a more distant peak (
). The vertical dashed line in
Figure 7 provides guidance for the relative positions of the minimum between features
and
.
In order to determine the types of Fe defects in the 15Fe-500 and 15Fe-1100 samples we simulated the Fe K-XANES spectra for each structure considered in
Section 3.3 and
Section 3.4.
Figure 7 shows the simulated spectra of the most probable defect structures.
Figure S2 in Supplementary Information shows all calculated spectra. The comparison of experimental and simulated spectra for magnetite Fe
O
(dotted curves in
Figure 7) reveals the main insufficiencies of the simulation method, including (i) the lack of pre-edge features (
) and (ii) a poor reproduction of the
satellite. However, the intensities and energy positions of the main features (
,
and
) of the magnetite spectrum are correctly reproduced. All spectral features of another reference iron oxide, hematite Fe
O
, could be reproduced (not shown) using exact the same calculation scheme. In this case, the differences between experimental and simulated spectra show qualitatively the same insufficiencies as for magnetite.
The simulated spectra for Fe-HAp show good sensitivity with respect to their atomistic structures and charge states. In most cases the increase of the iron oxidation state shifts the position of the main peak (
) to higher energies. This follows from an increase of the local positive charge and binding energy of K-electrons. An exception is seen for the Fe
spectrum (
Figure S2d), which is explained by significant changes in its local atomic structure (coordination change from 4 to 6) upon ionization.
The relative intensities and , corresponding to features and from the simulated spectra, are sensitive to the defect type: phosphorus substitutions have , calcium substitutions show , and most interstitial defects have . Additionally, the spectra show considerable differences concerning the position and shape of the feature.
The spectrum of the 15Fe-1100 sample shows comparable
and
intensities (
), suggesting that most Fe defects are of interstitial character. Conversely, the higher intensity of peak
in the spectrum of sample 15Fe-500 may indicate the presence of substitutional Fe on calcium sites or
defect (
,
). The latter scenario is in line with the conclusions of Gomes et al. [
18], where the formation of the high-symmetry Fe
structure was proposed in the 15Fe-500 and 15Fe-800 samples, whereas Fe
-type defects were suggested for the high-temperature treated 15Fe-1100 sample.