Efficient Recovery of Lithium Cobaltate from Spent Lithium-Ion Batteries for Oxygen Evolution Reaction
Abstract
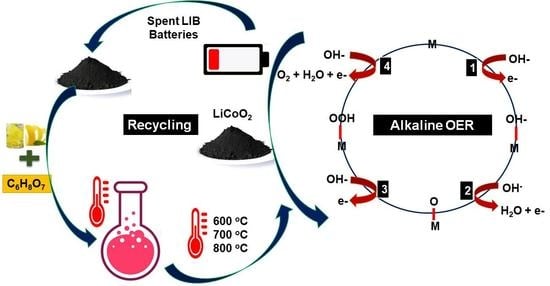
:1. Introduction
2. Experimental Section
2.1. Materials and Instruments
2.2. Metal Recovery and Sample Preparation
2.3. Reductive Leaching
2.4. Electrode Preparation
2.5. Analytical Methods
3. Results and Discussion
3.1. Metal Recovery
3.1.1. Temperature and Time
3.1.2. Acid Concentration and Extract Volume
3.2. Material Characterization
3.3. Electrocatalytic Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wang:, L.; Yin, F.; Yao, C. N-doped graphene as a bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions in an alkaline electrolyte. Int. J. Hydrog. Energy 2014, 39, 15913–15919. [Google Scholar] [CrossRef]
- Gong, M.; Dai, H. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 2015, 8, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Bizzotto, F.; Quinson, J.; Zana, A.; Kirkensgaard, J.J.K.; Dworzak, A.; Oezaslan, M.; Arenz, M. Ir nanoparticles with ultrahigh dispersion as oxygen evolution reaction (OER) catalysts: Synthesis and activity benchmarking. Catal. Sci. Technol. 2019, 9, 6345–6356. [Google Scholar] [CrossRef] [Green Version]
- Risch, M.; Stoerzinger, K.A.; Han, B.; Regier, T.Z.; Peak, D.; Sayed, S.Y.; Wei, C.; Xu, Z.; Shao-Horn, Y. Redox Processes of Manganese Oxide in Catalyzing Oxygen Evolution and Reduction: An in Situ Soft X-ray Absorption Spectroscopy Study. J. Phys. Chem. C 2017, 121, 17682–17692. [Google Scholar] [CrossRef] [Green Version]
- Fominykh, K.; Chernev, P.; Zaharieva, I.; Sicklinger, J.; Stefanic, G.; Döblinger, M.; Müller, A.; Pokharel, A.; Böcklein, S.; Scheu, C.; et al. Iron-Doped Nickel Oxide Nanocrystals as Highly Efficient Electrocatalysts for Alkaline Water Splitting. ACS Nano 2015, 9, 5180–5188. [Google Scholar] [CrossRef]
- Lankauf, K.; Cysewska, K.; Karczewski, J.; Mielewczyk-Gryń, A.; Górnicka, K.; Cempura, G.; Chend, M.; Jasińskia, P.; Molin, S. MnxCo3−xO4 spinel oxides as efficient oxygen evolution reaction catalysts in alkaline media. Int. J. Hydrog. Energy 2020, 45, 14867–14879. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Zhou, Q.; Wang, Q.; Zhang, X.; Tang, Y.; Sun, D. Coupling Hierarchical Ultrathin Co Nanosheets with N-doped Carbon Plate as High-Efficiency Oxygen Evolution Electrocatalysts. Front. Nanotechnol. 2021, 3, 17. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Yang, B.; Zhou, Y.; Zhang, Z.; Wang, Y. Photo-deposition of ZnO/Co3O4 core-shell nanorods with pn junction for efficient oxygen evolution reaction. J. Solid State Electrochem. 2019, 23, 3287–3297. [Google Scholar] [CrossRef]
- Zhu, S.; He, W.Z.; Li, G.M.; Xu, Z.; Zhang, X.; Huang, J. Recovery of Co and Li from spent lithium-ion batteries by combination method of acid leaching and chemical precipitation. Trans. Nonferrous Met. Soc. China 2012, 22, 2274–2281. [Google Scholar] [CrossRef]
- Lewis, C.; Li, Y.; Wang, L.; Li, J.; Stach, E.; Takeuchi, K.J.; Marschilok, A.C.; Takeuchi, E.S.; Wong, S.S. Correlating Titania Nanostructured Morphologies with Performance as Anode Materials for Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2016, 4, 6299–6312. [Google Scholar] [CrossRef]
- Ordoñez, J.; Gago, E.J.; Girard, A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew. Sustain. Energy Rev. 2016, 60, 195–205. [Google Scholar] [CrossRef]
- Ferreira, D.A.; Prados, L.; Majuste, D.; Mansur, M. Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries. J. Power Sources 2009, 187, 238–246. [Google Scholar] [CrossRef]
- Lee, S.W.; Carlton, C.; Risch, M.; Surendranath, Y.; Chen, S.; Furutsuki, S.; Yamada, A.; Nocera, D.G.; Shao-Horn, Y. The Nature of Lithium Battery Materials under Oxygen Evolution Reaction Conditions. J. Am. Chem. Soc. 2012, 134, 16959–16962. [Google Scholar] [CrossRef]
- Garcia, E.M.; Freitas, V.; Lins, C.; Tarôco, H.A.; Matencio, T.; Domingues, R.Z.; dos Santos, J.A. The anode environmentally friendly for water electrolysis based in LiCoO2 recycled from spent lithium-ion batteries. Int. J. Hydrog. Energy 2012, 37, 16795–16799. [Google Scholar] [CrossRef]
- Chen, N.; Qi, J.; Du, X.; Wang, Y.; Zhang, W.; Wang, Y.; Lu, Y.; Wang, S. Recycled LiCoO2 in spent lithium-ion battery as an oxygen evolution electrocatalyst. RSC Adv. 2016, 6, 103541–103545. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, H.; Kong, D.; Yan, K.; Hsu, P.; Zheng, G.; Yao, H.; Liang, Z.; Sun, X.; Cui, Y. Electrochemical tuning of layered lithium transition metal oxides for improvement of oxygen evolution reaction. Nat. Commun. 2014, 5, 4345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EPA. Environmental Protection Agency. Available online: https://www.epa.gov/recycle/used-lithium-ion-batteries (accessed on 12 November 2021).
- U.S. Geological Survey. Mineral Commodity Summaries 2021. Available online: https://pubs.er.usgs.gov/publication/mcs2021 (accessed on 11 November 2021).
- Statista Research Department. Cobalt Monthly Futures Price 2021. 4 October 2021. Available online: https://0-www-statista-com.brum.beds.ac.uk/statistics/1171975/global-monthly-price-of-cobalt/ (accessed on 10 November 2021).
- Vargas., P. Lithium and the Foreseeable Future. Bachelor’s Thesis, University of Arkansas, Fayetteville, AR, USA, 2018. [Google Scholar]
- Lithium and Cobalt CBS September 2021—Lithium Price Surges, Cobalt Range-Bound. Available online: https://www.spglobal.com/marketintelligence/en/news-insights/blog/lithium-and-cobalt-cbs-september-2021-lithium-price-surges-cobalt-range-bound (accessed on 10 November 2021).
- Eason, E. World Lithium Supply; Stanford University: Stanford, CA, USA, 2010. [Google Scholar]
- Zheng, X.; Gao, W.; Zhang, X.; He, M.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Spent lithium-ion battery recycling–Reductive ammonia leaching of metals from cathode scrap by sodium sulphite. Waste Manag. 2017, 60, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. Development of a recycling process for Li-ion batteries. J. Power Sources 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, J.; Li, H.; Chen, Y.; Wang, C. A promising approach for the recovery of high value-added metals from spent lithium-ion batteries. J. Power Sources 2017, 351, 192–199. [Google Scholar] [CrossRef]
- Takacova, Z.; Havlik, T.; Kukurugya, F.; Orac, D. Cobalt and lithium recovery from active mass of spent Li-ion batteries: Theoretical and experimental approach. Hydrometallurgy 2016, 163, 9–17. [Google Scholar] [CrossRef]
- Pinna, E.G.; Ruiz, M.C.; Ojeda, M.W.; Rodriguez, M.H. Cathodes of spent Li-ion batteries: Dissolution with phosphoric acid and recovery of lithium and cobalt from leach liquors. Hydrometallurgy 2017, 167, 66–71. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical processes for recycling spent lithium-ion batteries: A critical review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Gao, W.; Song, J.; Cao, H.; Lin, X.; Zhang, X.; Zheng, X.; Zhang, Y.; Sun, Z. Selective recovery of valuable metals from spent lithium-ion batteries–process development and kinetics evaluation. J. Clean. Prod. 2018, 178, 833–845. [Google Scholar] [CrossRef]
- Chen, X.; Fan, B.; Xu, L.; Zhou, T.; Kong, J. An atom-economic process for the recovery of high value-added metals from spent lithium-ion batteries. J. Clean. Prod. 2016, 112, 3562–3570. [Google Scholar] [CrossRef]
- Eaks, I.L. Ascorbic Acid Content of Citrus During Growth and Development. Bot. Gaz. 1964, 125, 186–191. [Google Scholar] [CrossRef]
- Sir Elkhatim, K.A.; Elagib, R.A.; Hassan, A.B. Content of phenolic compounds and vitamin C and antioxidant activity in wasted parts of Sudanese citrus fruits. Food Sci. Nutr. 2018, 6, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, J.; Ren, Y.; Zhang, X.; Chen, R.; Wu, F.; Amine, K. Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries. J. Power Sources 2012, 218, 21–27. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Zhang, Y.; Dong, P.; Wang, D.; Zhou, Z.; Duan, J.; Li, X.; Lin, Y.; Meng, Q.; Pai, K.V.; et al. An environmental friendly attempt to recycle the spent Li-ion battery cathode through organic acid leaching. J. Environ. Chem. Eng. 2019, 7, 102854. [Google Scholar] [CrossRef]
- Li, J.; Zhao, R.; He, X.; Liu, H. Preparation of LiCoO2 cathode materials from spent lithium–ion batteries. Ionics 2009, 15, 111–113. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Wu, F.; Chen, R.; Chen, S.; Wu, B. Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. J. Hazard. Mater. 2010, 176, 288–293. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Manjanna, J.; Pai, K.V.; Vadavi, R.; Keny, S.J.; Tripathi, V.S. Recovery of valuable metal ions from the spent lithium-ion battery using aqueous mixture of mild organic acids as alternative to mineral acids. Hydrometallurgy 2015, 151, 73–77. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, T. Hydrometallurgical process for the recovery of metal values from spent lithium-ion batteries in citric acid media. Waste Manag. Res. 2014, 32, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Zhang, Z.; Xue, F.; Yang, B. A more simple and efficient process for recovery of cobalt and lithium from spent lithium-ion batteries with citric acid. Sep. Purif. Technol. 2019, 215, 398–402. [Google Scholar]
- Zhuravlev, V.; Shikhovtseva, A.; Ermakova, L.; Evshchik, E.; Sherstobitova, E.; Novikov, D.; Bushkova, O.V.; Dobrovolsky, Y.A. Solution combustion synthesis of lithium cobalt oxide–cathode material for lithium-ion batteries. Int. J. Electrochem. Sci. 2019, 14, 2965–2983. [Google Scholar] [CrossRef]
- Xia, H.; Wan, Y.; Assenmacher, W.; Mader, W.; Yuan, G.; Lu, L. Facile synthesis of chain-like LiCoO2 nanowire arrays as three-dimensional cathode for microbatteries. NPG Asia Mater. 2014, 6, e126. [Google Scholar] [CrossRef] [Green Version]
- Qi, Z.; Koenig, G.M., Jr. High-performance LiCoO2 sub-micrometer materials from scalable microparticle template processing. ChemistrySelect 2016, 1, 3992–3999. [Google Scholar] [CrossRef]
- Manigandan, R.; Giribabu, K.; Suresh, R.; Vijayalakshmi, L.; Stephen, A.; Narayanan, V. Cobalt oxide nanoparticles: Characterization and its electrocatalytic activity towards nitrobenzene. Chem. Sci. Trans. 2013, 2, S47–S50. [Google Scholar]
- Freitas, M.B.; Garcia, E.M. Electrochemical recycling of cobalt from cathodes of spent lithium-ion batteries. J. Power Sources 2007, 171, 953–959. [Google Scholar] [CrossRef]
- Abdel-Ghany, A.E.; Eid, A.E.; Salman, A.A.; Sharaby, C.M.; Abdel-Hamied, S.K. Synthesis and Structural Characterization of Lithiated and Delithiated LiCoO2 Using Different Chelating Agents. Egypt. J. Chem. 2010, 53, 417–434. [Google Scholar]
- Soltanmohammad, S.; Asgari, S. Characterization of LiCoO2. Nanopowders Produced by Sol-Gel Processing. J. Nanomater. 2010, 2010, 104012. [Google Scholar] [CrossRef] [Green Version]
- Mossali, E.; Picone, N.; Gentilini, L.; Rodrìguez, O.; Pérez, J.M.; Colledani, M. Lithium-ion batteries towards circular economy: A literature review of opportunities and issues of recycling treatments. J. Environ. Manag. 2020, 264, 110500. [Google Scholar] [CrossRef]
- Li, G.; Chuang, P. Identifying the forefront of electrocatalytic oxygen evolution reaction: Electronic double layer. Appl. Catal. B Environ. 2018, 239, 425–432. [Google Scholar] [CrossRef]
- Habibi, A.; Jalaly, M.; Rahmanifard, R.; Ghorbanzadeh, M. The effect of calcination conditions on the crystal growth and battery performance of nanocrystalline Li (Ni 1/3 Co 1/3 Mn 1/3) O2 as a cathode material for Li-ion batteries. New J. Chem. 2018, 42, 19026–19033. [Google Scholar] [CrossRef]
- Pegoretti, V.C.B.; Dixini, P.V.M.; Magnago, L.; Rocha, A.K.S.; Lelis, M.F.F.; Freitas, M.B.J.G. High-temperature (HT) LiCoO2 recycled from spent lithium ion batteries as catalyst for oxygen evolution reaction. Mater. Res. Bull. 2019, 110, 97–101. [Google Scholar] [CrossRef]
- Liu, P. Recycling Waste Batteries: Recovery of Valuable Resources or Reutilization as Functional Materials. ACS Sustain. Chem. Eng. 2018, 6, 11176–11185. [Google Scholar] [CrossRef]
- Park, K.R.; Jeon, J.E.; Ali, G.; Ko, Y.; Lee, J.; Han, H.; Mhin, S. Oxygen Evolution Reaction of Co-Mn-O Electrocatalyst Prepared by Solution Combustion Synthesis. Catalysts 2019, 9, 564. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Du, N.; Zheng, W.; Li, X.; Dai, Y.; He, G. Ultrathin Ni-Co double hydroxide nanosheets with conformal graphene coating for highly active oxygen evolution reaction and lithium ion battery anode materials. Chem. Eng. J. 2017, 327, 9–17. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database. 2012. Available online: https://srdata.nist.gov/xps/ (accessed on 20 June 2019).







| BET Surface Area (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) | |
|---|---|---|---|
| CP1 | 16.3016 | 17.53716 | 0.071471 |
| CP2 | 5.1648 | 10.98172 | 0.014216 |
| CP3 | 4.8027 | 11.57406 | 0.013897 |
| CP4 | 2.5255 | 6.68136 | 0.004218 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arif, A.; Xu, M.; Rashid, J.; Saraj, C.S.; Li, W.; Akram, B.; Hu, B. Efficient Recovery of Lithium Cobaltate from Spent Lithium-Ion Batteries for Oxygen Evolution Reaction. Nanomaterials 2021, 11, 3343. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123343
Arif A, Xu M, Rashid J, Saraj CS, Li W, Akram B, Hu B. Efficient Recovery of Lithium Cobaltate from Spent Lithium-Ion Batteries for Oxygen Evolution Reaction. Nanomaterials. 2021; 11(12):3343. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123343
Chicago/Turabian StyleArif, Ayesha, Ming Xu, Jamshaid Rashid, Chaudry Sajed Saraj, Wei Li, Bilal Akram, and Binbin Hu. 2021. "Efficient Recovery of Lithium Cobaltate from Spent Lithium-Ion Batteries for Oxygen Evolution Reaction" Nanomaterials 11, no. 12: 3343. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123343