Preparation and Characterization of Silver-Iron Bimetallic Nanoparticles on Activated Carbon Using Plasma in Liquid Process
Abstract
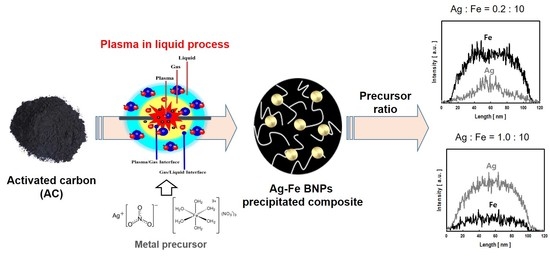
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Device
2.3. Preparation of SIACCs
2.4. Characterization of SIACCs
3. Results and Discussion
3.1. Characteristics of Aqueous Reactant Solution
3.2. Properties of Silver-Iron Bimetallic Nanoparticles
3.3. Controlling the Chemical Composition of Bimetallic Nanoparticle
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Ren, Z.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Hatzell, M.C.; Zhang, F.; Logan, B.L. Different electrode configurations to optimize performance of multi-electrode microbial fuel cells for generating power or treating domestic wastewater. J. Power Sources 2014, 249, 440–445. [Google Scholar] [CrossRef]
- Das, P.; Dutta, T.; Manna, S.; Loganathan, S.; Basak, P. Facile green synthesis of non-genotoxic, non-hemolytic organometallic silver nanoparticles using extract of crushed, wasted, and spent humulus lupulus (hops): Characterization, anti-bacterial, and anti-cancer studies. Environ. Res. 2022, 204, 111962. [Google Scholar] [CrossRef]
- Singh, N.; Siddiqui, H.; Kumar, S.; Goswami, M.; Kumar, A.; Sharda, T.; Kumar, S.; Sathish, N.; Srivastava, A.K. Electrochemical 3D printed silver nanoparticles for pharmaceutical drugs investigations. Mater. Lett. 2022, 307, 130976. [Google Scholar] [CrossRef]
- Gil-Díaz, M.; Rodríguez-Alonso, J.; Maffiotte, C.A.; Baragano, D.; Millan, R.; Lobo, M.C. Iron nanoparticles are efficient at removing mercury from polluted waters. J. Clean. Prod. 2021, 315, 128272. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Lao, Y.; Wan, P.; Mao, X.; Chen, F. Efficient magnetic harvesting of microalgae enabled by surface-initiated formation of iron nanoparticles. Chem. Eng. J. 2021, 408, 127252. [Google Scholar] [CrossRef]
- Ma, M.; You, S.; Gong, X.; Dai, Y.; Zou, J.; Fu, H. Silver/iron oxide/graphitic carbon composites as bacteriostatic catalysts for enhancing oxygen reduction in microbial fuel cells. J. Power Sources 2015, 283, 74–83. [Google Scholar] [CrossRef]
- Li, F.; Fu, L.; Li, J.; Yan, J.; Tang, Y.; Pan, Y.; Wang, H. Ag/Fe3O4-N-doped ketjenblack carbon composite as highly efficient oxygen reduction catalyst in al-air batteries. J. Electrochem. Soc. 2017, 164, A3595–A3601. [Google Scholar] [CrossRef]
- Khilari, S.; Pandit, S.; Ghangrekar, M.M.; Pradhan, D.; Das, D. Graphene oxide-impregnated PVA-STA composite polymer electrolyte membrane separator for power generation in a single-chambered microbial fuel cell. Ind. Eng. Chem. Res. 2013, 52, 11597–11606. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Cheng, S.; Huang, X.; Logan, B.E. The use of nylon and glass fiber filter separators with different pore sizes in air-cathode single-chamber microbial fuelcells. Energy Environ. Sci. 2010, 3, 659–664. [Google Scholar] [CrossRef]
- Chen, G.; Wei, B.; Luo, Y.; Logan, B.E.; Hickner, M.A. Polymer separators for high-power, high-efficiency microbial fuel cells. ACS Appl. Mater. Interfaces 2013, 4, 6454–6457. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.T.; Liu, Y.; Baek, J.; Dai, L.M. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef]
- Gnanakumar, G.; Awan, Z.; Nahm, K.S.; Xavier, J.S. Nanotubular MnO2/graphene oxide composites for the application of open air-breathing cathode microbial fuel cells. Biosens. Bioelectron. 2014, 53, 528–534. [Google Scholar] [CrossRef]
- Su, Y.H.; Jiang, H.L.; Zhu, Y.H.; Zou, W.J.; Yang, X.L.; Chen, J.D.; Li, C.Z. Hierarchical porous iron and nitrogen co-doped carbons as efficient oxygen reduction electrocatalysts in neutral media. J. Power Sources 2014, 265, 246–253. [Google Scholar] [CrossRef]
- Lee, H.; Park, S.H.; Seo, S.G.; Kim, S.J.; Kim, S.C.; Park, Y.K.; Jung, S.C. Preparation and characterization of copper nanoparticles via the liquid phase plasma method. Curr. Nanosci. 2014, 10, 7–10. [Google Scholar] [CrossRef]
- Kim, S.C.; Park, Y.K.; Jung, S.C. Recent applications of the liquid phase plasma process. Korean J. Chem. Eng. 2021, 38, 885–898. [Google Scholar] [CrossRef]
- Lee, H.; Park, S.H.; Kim, S.J.; Park, Y.K.; Kim, B.J.; An, K.H.; Ki, S.J.; Jung, S.C. Synthesis of manganese oxide/activated carbon composites for supercapacitor application using a liquid phase plasma reduction system. Int. J. Hydrogen Energy 2015, 40, 754–759. [Google Scholar] [CrossRef]
- Ki, S.J.; Lee, H.; Park, Y.K.; Kim, S.J.; An, K.H.; Jung, S.C. Assessing the electrochemical performance of a supercapacitor electrode made of copper oxide and activated carbon using liquid phase plasma. Appl. Surf. Sci. 2018, 446, 243–249. [Google Scholar] [CrossRef]
- Chung, K.H.; Kim, B.J.; Park, Y.K.; Kim, S.C.; Jung, S.C. Photocatalytic properties of amorphous N-doped TiO2 photocatalyst under visible light irradiation. Catalysts 2021, 11, 1010. [Google Scholar] [CrossRef]
- Sansonetti, J.E.; Martin, W.C. Handbook of basic atomic spectroscopic data. J. Phys. Chem. Ref. Data 2005, 34, 1559–2259. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Lee, W.J.; Park, Y.K.; Ki, S.J.; Kim, B.J.; Jung, S.C. Liquid phase plasma synthesis of iron oxide nanoparticles on nitrogen-doped activated carbon resulting in nanocomposite for supercapacitor applications. Nanomaterials 2018, 8, 190. [Google Scholar] [CrossRef] [Green Version]
- Idris, N.; Lahna, K.; Fadhli; Ramli, M. Study on emission spectral lines of iron, Fe in Laser-Induced Breakdown Spectroscopy (LIBS) on soil samples. J. Phys. Conf. Ser. 2015, 846, 012020. [Google Scholar] [CrossRef] [Green Version]
- Al-Asfar, A.; Zaheer, Z.; Aazam, E.S. Eco-friendly green synthesis of Ag@Fe bimetallic nanoparticles: Antioxidant, antimicrobial and photocatalytic degradation of bromothymol blue. J. Photochem. Photobiol. B 2018, 185, 143–152. [Google Scholar] [CrossRef]
- Alzahrani, S.A.; Malik, M.A.; Al-Thabaiti, S.A.; Khan, Z. Seedless synthesis and efficient recyclable catalytic activity of Ag@Fe nanocomposites towards methyl orange. Appl. Nanosci. 2018, 8, 255–271. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.H.; Jung, S.C. Facile synthesis of bimetallic Ni-Cu nanoparticles using liquid phase plasma method. Korean J. Chem. Eng. 2016, 33, 1075–1079. [Google Scholar] [CrossRef]
- Das, R.; Sypu, V.S.; Paumo, H.K.; Bhaumik, M.; Maharaj, V.; Maity, A. Silver decorated magnetic nanocomposite (Fe3O4@PPy-MAA/Ag) as highly active catalyst towards reduction of 4-nitrophenol and toxic organic dyes. Appl. Catal. B 2019, 244, 546–558. [Google Scholar] [CrossRef]
- Song, K.; Lee, Y.; Jo, M.R.; Nam, K.M.; Kang, Y.M. Comprehensive design of carbon-encapsulated Fe3O4 nanocrystals and their lithium storage properties. Nanotechnology 2012, 23, 505401. [Google Scholar] [CrossRef]
- Li, M.; Xue, J. Integrated synthesis of nitrogen-doped mesoporous carbon from melamine resins with superior performance in supercapacitors. J. Phys. Chem. C 2014, 118, 2507–2517. [Google Scholar] [CrossRef]
- Karamanova, B.; Stoyanova, A.; Shipochka, M.; Veleva, S.; Stoyanova, R. Effect of alkaline-basic electrolytes on the capacitance performance of biomass-derived carbonaceous materials. Materials 2020, 13, 2941. [Google Scholar] [CrossRef]
- Sadri, R.; Hosseini, M.; Kazi, S.N.; Bagheri, S.; Zubir, N.; Solangi, K.H.; Zaharinie, T.; Badarudin, A. A bio-based, facile approach for the preparation of covalently functionalized carbon nanotubes aqueous suspensions and their potential as heat transfer fluids. J. Colloid Interface Sci. 2017, 504, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Devi, T.B.; Mohanta, D.; Ahmaruzzaman, M. Biomass derived activated carbon loaded silver nanoparticles: An effective nanocomposites for enhanced solar photocatalysis and antimicrobial activities. J. Ind. Eng. Chem. 2019, 76, 160–172. [Google Scholar] [CrossRef]
- Prieto, P.; Nistor, V.; Nouneh, K.; Oyama, M.; Abd-Lefdil, M.; Diaz, R. XPS study of silver, nickel and bimetallic silver–nickel nanoparticles prepared by seed-mediated growth. Appl. Surf. Sci. 2012, 258, 8807–8813. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Li, X.; Le, X.; Ma, J. Catalysis of the hydro-dechlorination of 4-chlorophenol and the reduction of 4-nitrophenol by Pd/Fe3O4@SiO2@m-SiO2. New J. Chem. 2015, 39, 6474–6481. [Google Scholar] [CrossRef]
- Kireeti, K.V.M.K.; Chandrakanth, G.; Kadam, M.M.; Jha, N. A sodium modified reduced graphene oxide–Fe3O4 nanocomposite for efficient lead (II) adsorption. RSC Adv. 2016, 6, 84825–84836. [Google Scholar] [CrossRef]
- Bhargava, G.; Gouzman, I.; Chun, C.M.; Ramanarayanan, T.A.; Bernasek, S.L. Characterization of the ‘‘native’’ surface thin film on pure polycrystalline iron: A high resolution XPS and TEM study. Appl. Surf. Sci. 2007, 253, 4322–4329. [Google Scholar] [CrossRef]
- Wan, L.; Yan, D.; Xu, X.; Li, J.; Lu, T.; Gao, Y.; Yao, Y.; Pan, L. Self-assembled 3D flower-like Fe3O4/C architecture with superior lithium ion storage performance. J. Mater. Chem. A 2018, 6, 24940–24948. [Google Scholar] [CrossRef]
- Li, Z.; Guan, Z.; Guan, Z.; Liang, C.; Yu, K. Effect of deep cryogenic activated treatment on hemp stem-derived carbon used as anode for lithium-ion batteries. Nanoscale Res. Lett. 2020, 15, 193. [Google Scholar] [CrossRef]
- Liu, X.Y.; Huang, M.; Ma, H.L.; Zhang, Z.Q.; Gao, J.M.; Zhu, Y.L.; Han, X.J.; Guo, X.Y. Preparation of a carbon-based solid acid catalyst by sulfonating activated carbon in a chemical reduction process. Molecules 2010, 15, 7188–7196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banua, J.; Han, J.I. Biogenesis of prism-like silver oxide nanoparticles using nappa cabbage extract and their p-nitrophenol sensing activity. Molecules 2020, 25, 2298. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.Y.; Mohammad, A.W.; Rohani, R.; Hairom, N.H.H. Development of a nanofiltration membrane for humic acid removal through the formation of polyelectrolyte multilayers that contain nanoparticles. Desalin. Water Treat. 2015, 57, 7627–7636. [Google Scholar] [CrossRef]







| Sample | Initial Conc. (mM) | Carbon | Oxygen | Silver | Iron | |
|---|---|---|---|---|---|---|
| AgNO3 | Fe(NO3)3 | wt.% | wt.% | wt.% | wt.% | |
| AC(YP-50F) | 0 | 10 | 96.4 ± 0.3 | 3.6 ± 0.3 | 0.0 ±0.0 | 0.0 ± 0.0 |
| SIACC-01 | 0.1 | 10 | 93.2 ± 0.2 | 4.6 ± 0.2 | 0.3 ± 0.1 | 2.0 ± 0.2 |
| SIACC-02 | 0.2 | 10 | 93.1 ± 0.4 | 4.4 ± 0.2 | 0.7 ± 0.1 | 1.7 ± 0.1 |
| SIACC-10 | 1.0 | 10 | 91.9 ± 0.4 | 4.3 ± 0.3 | 3.0 ± 0.2 | 0.8 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Park, J.; Park, Y.-K.; Kim, B.-J.; An, K.-H.; Kim, S.-C.; Jung, S.-C. Preparation and Characterization of Silver-Iron Bimetallic Nanoparticles on Activated Carbon Using Plasma in Liquid Process. Nanomaterials 2021, 11, 3385. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123385
Lee H, Park J, Park Y-K, Kim B-J, An K-H, Kim S-C, Jung S-C. Preparation and Characterization of Silver-Iron Bimetallic Nanoparticles on Activated Carbon Using Plasma in Liquid Process. Nanomaterials. 2021; 11(12):3385. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123385
Chicago/Turabian StyleLee, Heon, Jaegu Park, Young-Kwon Park, Byung-Joo Kim, Kay-Hyeok An, Sang-Chai Kim, and Sang-Chul Jung. 2021. "Preparation and Characterization of Silver-Iron Bimetallic Nanoparticles on Activated Carbon Using Plasma in Liquid Process" Nanomaterials 11, no. 12: 3385. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123385