Fabrication and Characterization of Novel Poly(d-Lactic Acid) Nanocomposite Membrane for Water Filtration Purpose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of CNW
2.3. Preparation of PDLA Membranes
2.4. Nanocellulose Characterization
2.5. PDLA Membranes Characterization
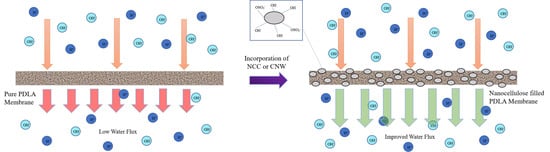
3. Results and Discussion
3.1. Morphology
3.2. Surface Wettability
3.3. Thermal Property
3.4. Tensile Property
3.5. Water Permeability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, D.; Yan, Y.; Wang, H. Recent advances in polymer and polymer composite membranes for reverse and forward osmosis processes. Prog. Polym. Sci. 2016, 61, 104–155. [Google Scholar] [CrossRef] [Green Version]
- Galiano, F.; Briceño, K.; Marino, T.; Molino, A.; Christensen, K.V.; Figoli, A. Advances in biopolymer-based membrane preparation and applications. J. Membr. Sci. 2018, 564, 562–586. [Google Scholar] [CrossRef]
- Minbu, H.; Ochiai, A.; Kawase, T.; Taniguchi, M.; Lloyd, D.R.; Tanaka, T. Preparation of poly(L-lactic acid) microfiltration membranes by a nonsolvent-induced phase separation method with the aid of surfactants. J. Membr. Sci. 2015, 479, 85–94. [Google Scholar] [CrossRef]
- Tanaka, T.; Nishimoto, T.; Tsukamoto, K.; Yoshida, M.; Kouya, T.; Taniguchi, M.; Lloyd, D.R. Formation of depth filter microfiltration membranes of poly(l-lactic acid) via phase separation. J. Membr. Sci. 2012, 396, 101–109. [Google Scholar] [CrossRef]
- Gokce, O.; Kasap, M.; Akpinar, G.; Ozkoc, G. Preparation, characterization, and in vitro evaluation of chicken feather fiber-thermoplastic polyurethane composites. J. Appl. Polym. Sci. 2017, 134, 45338. [Google Scholar] [CrossRef] [Green Version]
- Kiadeh, S.Z.H.; Ghaee, A.; Mashak, A.; Mohammadnejad, J. Preparation of chitosan-silica/PCL composite membrane as wound dressing with enhanced cell attachment. Polym. Adv. Technol. 2017, 28, 1396–1408. [Google Scholar] [CrossRef]
- Cai, Y.; Guo, J.; Chen, C.; Yao, C.; Chung, S.M.; Yao, J.; Lee, I.S.; Kong, X. Silk fibroin membrane used for guided bone tissue regeneration. Mater. Sci. Eng. C 2017, 70, 148–154. [Google Scholar] [CrossRef]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; Toit, L.C.; Ndesendo, V.M.K. A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J. Nanomater. 2013, 1, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic acid blends: The future of green, light and tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Chinyerenwa, A.C.; Wang, H.; Zhang, Q.; Zhuang, Y.; Munna, K.H.; Ying, C.; Yang, H.; Xu, W. Structure and thermal properties of porous polylactic acid membranes prepared via phase inversion induced by hot water droplets. Polymer 2018, 141, 62–69. [Google Scholar] [CrossRef]
- Gao, A.; Liu, F.; Shi, H.; Xue, L. Controllable transition from finger-like pores to inter-connected pores of PLLA membranes. J. Membr. Sci. 2015, 478, 96–104. [Google Scholar] [CrossRef]
- Phaechamud, T.; Chitrattha, S. Pore formation mechanism of porous poly(DL-lactic acid) matrix membrane. Mater. Sci. Eng. C 2016, 61, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Zhou, Y.; Zhang, L. Morphology and mechanical properties of a new nanocrystalline cellulose/polysulfone composite membrane. Adv. Polym. Technol. 2014, 34. [Google Scholar] [CrossRef]
- Zhong, L.; Ding, Z.; Li, B.; Zhang, L. Preparation and characterization of polysulfone/sulfonated polysulfone/cellulose nanofibers ternary blend membranes. Bioresources 2012, 10, 2936–2948. [Google Scholar] [CrossRef] [Green Version]
- Badawi, N.E.; Ramadan, A.R.; Esawi, A.M.K.; Morsi, M.E. Novel carbon nanotube–cellulose acetate nanocomposite membranes for water filtration applications. Desalination 2014, 344, 79–85. [Google Scholar] [CrossRef]
- Choi, H.; Yoon, S.H.; Son, M.; Celik, E.; Park, H.; Choi, H. Efficacy of synthesis conditions on functionalized carbon nanotube blended cellulose acetate membrane for desalination. Desalin. Water Treat. 2015, 57, 7545–7554. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Aroujalian, A.; Raisi, A. Effect of added NaX nano-zeolite into polyamide as a top thin layer of membrane on water flux and salt rejection in a reverse osmosis process. J. Membr. Sci. 2011, 375, 88–95. [Google Scholar] [CrossRef]
- Huang, H.; Qu, X.; Dong, H.; Zhang, L.; Chen, H. Role of NaA zeolites in the interfacial polymerization process towards a polyamide nanocomposite reverse osmosis membrane. RSC Adv. 2013, 3, 8203–8207. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent developments on nanocellulose reinforced polymer nanocomposites: A review. Polymer 2017, 132, 368–393. [Google Scholar] [CrossRef]
- Kian, L.K.; Jawaid, M.; Ariffin, H.; Karim, Z. Isolation and characterization of nanocrystalline cellulose from roselle-derived microcrystalline cellulose. Int. J. Biol. Macromol. 2018, 114, 54–63. [Google Scholar] [CrossRef]
- Li, Y.; Huang, S.; Zhou, S.; Fane, A.G.; Zhang, Y.; Zhao, S. Enhancing water permeability and fouling resistance of polyvinylidene fluoride membranes with carboxylated nanodiamonds. J. Membr. Sci. 2018, 556, 154–163. [Google Scholar] [CrossRef]
- Fan, T.; Li, Z.; Cheng, B.; Li, J. Preparation, characterization of PPS micro-porous membranes and their excellent performance in vacuum membrane distillation. J. Membr. Sci. 2018, 556, 107–117. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Song, M.H.; Lee, J.Y.; Lee, H.K.; Yoo, D.J. Amine functionalized carbon nanotube (ACNT) filled in sulfonated poly(ether ether ketone) membrane: Effects of ACNT in improving polymer electrolyte fuel cell performance under reduced relative humidity. Compos. Part B Eng. 2020, 188, 107890. [Google Scholar] [CrossRef]
- Kim, A.R.; Park, C.J.; Vinothkannan, M.; Yoo, D.J. Sulfonated poly ether sulfone/heteropoly acid composite membranes as electrolytes for the improved power generation of proton exchange membrane fuel cells. Compos. Part B Eng. 2018, 155, 272–281. [Google Scholar] [CrossRef]
- Ouyang, L.; Malaisamy, R.; Bruening, M.L. Multilayer polyelectrolyte films as nanofiltration membranes for separating monovalent and divalent cations. J. Membr. Sci. 2008, 310, 76–84. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.H.; Cho, J.; Bang, J. Desalination membranes from pH-controlled and thermally-crosslinked layer-by-layer assembled multilayers. J. Mater. Chem. 2010, 20, 2085–2091. [Google Scholar] [CrossRef]







| Membranes | Rq (nm) a | Spore (μm) b | CA (°) c | Pw (L/m2h) d |
|---|---|---|---|---|
| PD-neat | 0.0479 | 0.220 | 72.36 ± 0.26 | 39.26 |
| PDNC-I | 0.0350 | 0.156 | 70.63 ± 0.31 | 39.74 |
| PDNC-II | 0.0324 | 0.193 | 59.77 ± 0.54 | 40.98 |
| PDNW-I | 0.0278 | 0.117 | 59.07 ± 0.32 | 41.36 |
| PDNW-II | 0.0435 | 0.134 | 58.44 ± 0.44 | 41.92 |
| Membranes | Tonset (°C) a | Tpeak (°C) b | Ts (MPa) c | Eab (%) d | Ym (GPa) e |
|---|---|---|---|---|---|
| PD-neat | 312.9 | 350.3 | 22.3 | 6.5 | 0.7 |
| PDNC-I | 335.9 | 365.9 | 30.5 | 9.6 | 0.5 |
| PDNC-II | 317.4 | 355.4 | 39.2 | 5.4 | 1.3 |
| PDNW-I | 336.5 | 367.4 | 37.9 | 9.3 | 0.8 |
| PDNW-II | 318.1 | 357.2 | 25.4 | 8.4 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kian, L.K.; Jawaid, M.; Alamery, S.; Vaseashta, A. Fabrication and Characterization of Novel Poly(d-Lactic Acid) Nanocomposite Membrane for Water Filtration Purpose. Nanomaterials 2021, 11, 255. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11020255
Kian LK, Jawaid M, Alamery S, Vaseashta A. Fabrication and Characterization of Novel Poly(d-Lactic Acid) Nanocomposite Membrane for Water Filtration Purpose. Nanomaterials. 2021; 11(2):255. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11020255
Chicago/Turabian StyleKian, Lau Kia, Mohammad Jawaid, Salman Alamery, and Ashok Vaseashta. 2021. "Fabrication and Characterization of Novel Poly(d-Lactic Acid) Nanocomposite Membrane for Water Filtration Purpose" Nanomaterials 11, no. 2: 255. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11020255