Advances in Electrochemical Energy Devices Constructed with Tungsten Oxide-Based Nanomaterials
Abstract
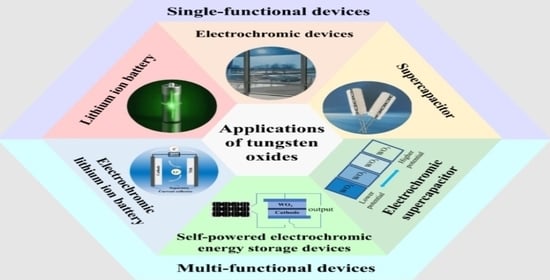
:1. Introduction
2. Energy Storage Mechanism of Tungsten Oxides
2.1. The Crystal Structure of Tungsten Oxides
2.2. Phase Transformation in Tungsten Oxides toward Energy Storage
3. Energy Storage Devices Based on Tungsten Oxides
3.1. WO3 Electrode Materials of Supercapacitors
3.1.1. Single Phase WO3 Nanostructures
3.1.2. Multi-Phased Tungsten Oxide Nanocomposites
3.2. Tungsten Oxide-Based Materials as Anodes in Lithium Ion Battery
3.2.1. Non-Stoichiometric Tungsten Oxides
3.2.2. Nano-Structured Tungsten Oxides
3.2.3. Tungsten Oxide-Carbon Composites
4. Electrochromic Applications
4.1. Tungsten Oxides as EC Electrode in Visible Light Area
4.2. Tungsten Oxides as EC Electrode in NIR Area
4.2.1. Inverse Opal-Structured Tungsten Oxides
4.2.2. Dynamic Control of Visible and NIR Light of Tungsten Oxide ECDs
5. Electrochromic Energy Storage Devices (ECESDs)
5.1. Tungsten Oxides Based ECESDs
5.2. Quantitative Judgement of Energy Level Function of Tungsten Oxide Based ECESDs
6. Solar Cell and Tungsten Oxide-Based EC Integrated Multifunctional Devices
7. Other Applications of Tungsten Oxides-Based Materials
8. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Divya, K.; Østergaard, J. Battery energy storage technology for power systems—An overview. Electr. Power Syst. Res. 2009, 79, 511–520. [Google Scholar] [CrossRef]
- Amrouche, S.O.; Rekioua, D.; Bacha, S. Overview of energy storage in renewable energy systems. Int. J. Hydrog. Energy 2016, 41, 20914–20927. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Baptista, J.M.; Sagu, J.S.; Kg, U.W.; Lobato, K. State-of-the-art materials for high power and high energy supercapacitors: Performance metrics and obstacles for the transition from lab to industrial scale—A critical approach. Chem. Eng. J. 2019, 374, 1153–1179. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Deb, S. Opportunities and challenges of electrochromic phenomena in transition metal oxides. Sol. Energy Mater. Sol. Cells 1992, 25, 327–338. [Google Scholar] [CrossRef]
- Granqvist, C. Handbook of Inorganic Electrochromic Materials; Elsevier Science: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Granqvist, C.G. Oxide electrochromics: An introduction to devices and materials. Sol. Energy Mater. Sol. Cells 2012, 99, 1–13. [Google Scholar] [CrossRef]
- Sharma, K.; Arora, A.; Tripathi, S. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Plenum Press: New York, NY, USA, 1999. [Google Scholar]
- Shukla, A.; Banerjee, A.; Ravikumar, M.; Jalajakshi, A. Electrochemical capacitors: Technical challenges and prognosis for future markets. Electrochim. Acta 2012, 84, 165–173. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Hu, R.; Liu, H.; Zeng, M.; Liu, J.; Zhu, M. Progress on Sn-based thin-film anode materials for lithium-ion batteries. Chin. Sci. Bull. 2012, 57, 4119–4130. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.; Zhang, H.; Bu, Y.; Zhang, H.; Zhao, B.; Yang, C. Porous Co3O4 nanofibers surface-modified by reduced graphene oxide as a durable, high-rate anode for lithiumion battery. Electrochim. Acta 2017, 228, 241–250. [Google Scholar] [CrossRef]
- Li, W.-J.; Fu, Z.-W. Nanostructured WO3 thin film as a new anode material for lithium-ion batteries. Appl. Surf. Sci. 2010, 256, 2447–2452. [Google Scholar] [CrossRef]
- Monk, P.; Mortimer, R.; Rosseinsky, D. Electrochromism and Electrochromic Devices; Cambridge University Press: New York, NY, USA, 2007. [Google Scholar]
- Pérez-Lombard, L.; Ortiz, J.; Pout, C. A review on buildings energy consumption information. Energy Build. 2008, 40, 394–398. [Google Scholar] [CrossRef]
- Azens, A.; Granqvist, C.G. Electrochromic smart windows: Energy efficiency and device aspects. J. Solid State Electrochem. 2003, 7, 64–68. [Google Scholar] [CrossRef]
- Tang, W.; Liu, L.; Tian, S.; Li, L.; Yue, Y.; Wu, Y.; Zhu, K. Aqueous supercapacitors of high energy density based on MoO3 nanoplates as anode material. Chem. Commun. 2011, 47, 10058–10060. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, Y.; Sang, L.; Ma, J.; Shi, H.; Liu, X.; Lu, J.; Zhang, Y. Boosting the electrochemical performance of MoO3 anode for long-life lithium ion batteries: Dominated by an ultrathin TiO2 passivation layer. Electrochim. Acta 2018, 269, 241–249. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, Y.; Jin, D.; Xie, Y. Novel Metastable Hexagonal MoO3Nanobelts: Synthesis, Photochromic, and Electrochromic Properties. Chem. Mater. 2009, 21, 5681–5690. [Google Scholar] [CrossRef]
- Liu, K.-Y.; Zhang, Y.; Zhang, W.; Zheng, H.; Su, G. Charge-discharge process of MnO2 supercapacitor. Trans. Nonferrous Met. Soc. China 2007, 17, 649–653. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; He, X.; Xu, S.; Fang, M.; Zhao, X.; Shang, Y. Electrochemical properties of MnO2 nanorods as anode materials for lithium ion batteries. Electrochim. Acta 2014, 142, 152–156. [Google Scholar] [CrossRef]
- Sakai, N.; Ebina, Y.; Takada, K.; Sasaki, T. Electrochromic Films Composed of MnO2 Nanosheets with Controlled Optical Density and High Coloration Efficiency. J. Electrochem. Soc. 2005, 152, E384–E389. [Google Scholar] [CrossRef]
- Granqvist, C. Electrochromic tungsten oxide films: Review of progress 1993–1998. Sol. Energy Mater. Sol. Cells 2000, 60, 201–262. [Google Scholar] [CrossRef]
- Zhu, M.; Meng, W.; Huang, Y.; Zhi, C. Proton-Insertion-Enhanced Pseudocapacitance Based on the Assembly Structure of Tungsten Oxide. ACS Appl. Mater. Interfaces 2014, 6, 18901–18910. [Google Scholar] [CrossRef]
- Deb, S.K. A Novel Electrophotographic System. Appl. Opt. 1969, 8, 192–195. [Google Scholar] [CrossRef]
- Yun, T.G.; Park, M.; Kim, D.-H.; Kim, D.; Cheong, J.Y.; Bae, J.G.; Han, S.M.; Kim, I.-D. All-Transparent Stretchable Electrochromic Supercapacitor Wearable Patch Device. ACS Nano 2019, 13, 3141–3150. [Google Scholar] [CrossRef]
- Cannavale, A.; Manca, M.; Malara, F.; De Marco, L.; Cingolani, R.; Gigli, G. Highly efficient smart photovoltachromic devices with tailored electrolyte composition. Energy Environ. Sci. 2011, 4, 2567–2574. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, B.; Huang, H.; Gan, Y.; Xia, Y.; Liang, C.; Zhang, W.; Zhang, J. A Solar-Driven Flexible Electrochromic Supercapacitor. Materials 2020, 13, 1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, Z.; Li, X.; Chen, Y.; He, X.; Xu, X.; Gao, X. Large-Scale Multifunctional Electrochromic-Energy Storage Device Based on Tungsten Trioxide Monohydrate Nanosheets and Prussian White. ACS Appl. Mater. Interfaces 2017, 9, 29872–29880. [Google Scholar] [CrossRef] [PubMed]
- Buch, V.R.; Chawla, A.K.; Rawal, S.K. Review on electrochromic property for WO3 thin films using different deposition techniques. Mater. Today Proc. 2016, 3, 1429–1437. [Google Scholar] [CrossRef]
- Dong, P.; Hou, G.; Xi, X.; Shao, R.; Dong, F. WO3-based photocatalysts: Morphology control, activity enhancement and multifunctional applications. Environ. Sci. Nano 2017, 4, 539–557. [Google Scholar] [CrossRef]
- Hariharan, V.; Gnanavel, B.; Sathiyapriya, R.; Aroulmoji, V. A Review on Tungsten Oxide (WO3) and their Derivatives for Sensor Applications. Int. J. Adv. Sci. Eng. 2019, 5, 1163–1168. [Google Scholar] [CrossRef]
- Vogt, T.; Woodward, P.M.; Hunter, B.A. The High-Temperature Phases of WO3. J. Solid State Chem. 1999, 144, 209–215. [Google Scholar] [CrossRef]
- Rao, M. Structure and properties of WO3 thin films for electrochromic device application. J. Non-Oxide Glas. 2013, 5, 1–8. [Google Scholar]
- Ramana, C.V.; Utsunomiya, S.; Ewing, R.C.; Julien, C.; Becker, U. Structural Stability and Phase Transitions in WO3 Thin Films. J. Phys. Chem. B 2006, 110, 10430–10435. [Google Scholar] [CrossRef]
- Gerand, B.; Nowogrocki, G.; Guenot, J.; Figlarz, M. Structural study of a new hexagonal form of tungsten trioxide. J. Solid State Chem. 1979, 29, 429–434. [Google Scholar] [CrossRef]
- Sun, W.; Yeung, M.T.; Lech, A.T.; Lin, C.-W.; Lee, C.; Li, T.; Duan, X.; Zhou, J.; Kaner, R.B. High Surface Area Tunnels in Hexagonal WO3. Nano Lett. 2015, 15, 4834–4838. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Djaoued, Y.; Albert, A.-S.; Ferguson, R.Z.; Brüning, R. Hexagonal Tungsten Oxide Based Electrochromic Devices: Spectroscopic Evidence for the Li Ion Occupancy of Four-Coordinated Square Windows. Chem. Mater. 2009, 21, 1381–1389. [Google Scholar] [CrossRef]
- Roussel, P.; Labbé, P.; Groult, D. Symmetry and twins in the monophosphate tungsten bronze series (PO2)4(WO3)2m (2 ≤ m ≤ 14). Acta Crystallogr. Sect. B Struct. Sci. 2000, 56, 377–391. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, H.; Liu, J.; Zhang, Q.; Yan, H. Nonstoichiometric tungsten oxide: Structure, synthesis, and applications. J. Mater. Sci. Mater. Electron. 2019, 31, 861–873. [Google Scholar] [CrossRef]
- Makarov, V.; Trontelj, M. Sintering and electrical conductivity of doped WO3. J. Eur. Ceram. Soc. 1996, 16, 791–794. [Google Scholar] [CrossRef]
- Sahle, W.; Nygren, M. Electrical conductivity and high resolution electron microscopy studies of WO3−x crystals with 0 ≤ x ≤ 0.28. J. Solid State Chem. 1983, 48, 154–160. [Google Scholar] [CrossRef]
- Liao, C.-C.; Chen, F.-R.; Kai, J.-J. Annealing effect on electrochromic properties of tungsten oxide nanowires. Sol. Energy Mater. Sol. Cells 2007, 91, 1258–1266. [Google Scholar] [CrossRef]
- Augustyn, V.; Gogotsi, Y. 2D Materials with Nanoconfined Fluids for Electrochemical Energy Storage. Joule 2017, 1, 443–452. [Google Scholar] [CrossRef]
- Judeinstein, P.; Livage, J. Role of the water content on the electrochromic properties of WO3, thin films. Mater. Sci. Eng. B 1989, 3, 129–132. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, J.; Zhou, Y.; Xie, J.; Zhang, X.; Guan, M.; Pan, B.; Xie, Y. High-performance flexible electrochromic device based on facile semiconductor-to-metal transition realized by WO3·2H2O ultrathin nanosheets. Sci. Rep. 2013, 3, e01936. [Google Scholar] [CrossRef] [Green Version]
- Shinde, P.A.; Seo, Y.; Ray, C.; Jun, S.C. Direct growth of WO3 nanostructures on multi-walled carbon nanotubes for high-performance flexible all-solid-state asymmetric supercapacitor. Electrochim. Acta 2019, 308, 231–242. [Google Scholar] [CrossRef]
- He, X.; Wang, X.; Sun, B.; Wan, J.; Wang, Y.; He, D.; Suo, H.; Zhao, C. Synthesis of three-dimensional hierarchical furball-like tungsten trioxide microspheres for high performance supercapacitor electrodes. RSC Adv. 2020, 10, 13437–13441. [Google Scholar] [CrossRef] [Green Version]
- Zheng, F.; Wang, J.; Liu, W.; Zhou, J.; Li, H.; Yu, Y.; Hu, P.; Yan, W.; Liu, Y.; Li, R.; et al. Novel diverse-structured h-WO3 nanoflake arrays as electrode materials for high performance supercapacitors. Electrochim. Acta 2020, 334, 135641. [Google Scholar] [CrossRef]
- Jia, J.; Liu, X.; Mi, R.; Liu, N.; Xiong, Z.; Yuan, L.; Wang, C.; Sheng, G.; Cao, L.; Zhou, X.; et al. Self-assembled pancake-like hexagonal tungsten oxide with ordered mesopores for supercapacitors. J. Mater. Chem. A 2018, 6, 15330–15339. [Google Scholar] [CrossRef]
- Li, C.; Hsieh, J.; Su, T.; Wu, P. Experimental study on property and electrochromic function of stacked WO3/Ta2O5/NiO films by sputtering. Thin Solid Films 2018, 660, 373–379. [Google Scholar] [CrossRef]
- Chen, P.-W.; Chang, C.-T.; Ko, T.-F.; Hsu, S.-C.; Li, K.-D.; Wu, J.-Y. Fast response of complementary electrochromic device based on WO3/NiO electrodes. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Xie, S.; Chen, Y.; Bi, Z.; Jia, S.; Guo, X.; Gao, X.; Li, X. Energy storage smart window with transparent-to-dark electrochromic behavior and improved pseudocapacitive performance. Chem. Eng. J. 2019, 370, 1459–1466. [Google Scholar] [CrossRef]
- Mjejri, I.; Gaudon, M.; Rougier, A. Mo addition for improved electrochromic properties of V2O5 thick films. Sol. Energy Mater. Sol. Cells 2019, 198, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Zhang, H.; Chen, G.; Tian, T.; Tao, K.; Liang, L.; Gao, J.; Cao, H. Long-term-stable WO3-PB complementary electrochromic devices. J. Alloys Compd. 2021, 861, 158534. [Google Scholar] [CrossRef]
- Pham, N.S.; Seo, Y.H.; Park, E.; Nguyen, T.D.D.; Shin, I.-S. Implementation of high-performance electrochromic device based on all-solution-fabricated Prussian blue and tungsten trioxide thin film. Electrochim. Acta 2020, 353, 136446. [Google Scholar] [CrossRef]
- Zhong, Y.; Chai, Z.; Liang, Z.; Sun, P.; Xie, W.; Zhao, C.; Mai, W. Electrochromic Asymmetric Supercapacitor Windows Enable Direct Determination of Energy Status by the Naked Eye. ACS Appl. Mater. Interfaces 2017, 9, 34085–34092. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Lu, T.; Chang, X.; Hu, X.; Dong, L.; Yin, Y. Flexible electrochromic device based on WO3·H2O nanoflakes synthesized by a facile sonochemical method. Mater. Lett. 2016, 185, 319–322. [Google Scholar] [CrossRef]
- Ma, D.; Li, T.; Xu, Z.; Wang, L.; Wang, J. Electrochromic devices based on tungsten oxide films with honeycomb-like nanostructures and nanoribbons array. Sol. Energy Mater. Sol. Cells 2018, 177, 51–56. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, G.; Xiao, Y.; Gao, F.; Wang, M.; Wang, Q.; Wang, S.; Zuo, H.; Diao, X. An all-thin-film inorganic electrochromic device monolithically fabricated on flexible PET/ITO substrate by magnetron sputtering. Mater. Lett. 2015, 142, 232–234. [Google Scholar] [CrossRef]
- Yue, Y.; Li, H.; Li, K.; Wang, J.; Wang, H.; Zhang, Q.; Li, Y.; Chen, P. High-performance complementary electrochromic device based on WO 3 ·0.33H 2 O/PEDOT and prussian blue electrodes. J. Phys. Chem. Solids 2017, 110, 284–289. [Google Scholar] [CrossRef]
- Hashimoto, S.; Matsuoka, H. Mechanism of electrochromism for amorphous WO3thin films. J. Appl. Phys. 1991, 69, 933–937. [Google Scholar] [CrossRef]
- Hersh, H.N.; Kramer, W.E.; McGee, J.H. Mechanism of electrochromism in WO3. Appl. Phys. Lett. 1975, 27, 646–648. [Google Scholar] [CrossRef]
- Qiu, D.; Ji, H.; Zhang, X.; Zhang, H.; Cao, H.; Chen, G.; Tian, T.; Chen, Z.; Guo, X.; Liang, L.; et al. Electrochromism of Nanocrystal-in-Glass Tungsten Oxide Thin Films under Various Conduction Cations. Inorg. Chem. 2019, 58, 2089–2098. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zheng, X.; Zhang, X.; Xiao, H.; Qu, F.; Wu, X. Facile synthesis of flexible WO3 nanofibers as supercapacitor electrodes. Mater. Lett. 2017, 186, 94–97. [Google Scholar] [CrossRef]
- Wang, R.; Lu, Y.; Zhou, L.; Han, Y.; Ye, J.; Xu, W.; Lu, X.; Lu, Y. Oxygen-deficient tungsten oxide nanorods with high crystallinity: Promising stable anode for asymmetric supercapacitors. Electrochim. Acta 2018, 283, 639–645. [Google Scholar] [CrossRef]
- Yin, Z.; Bu, Y.; Ren, J.; Chen, S.; Zhao, D.; Zou, Y.; Shen, S.; Yang, D. Triggering superior sodium ion adsorption on (2 0 0) facet of mesoporous WO3 nanosheet arrays for enhanced supercapacitance. Chem. Eng. J. 2018, 345, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Yao, S. Flexible electrode materials based on WO3 nanotube bundles for high performance energy storage devices. Nano Energy 2017, 42, 143–150. [Google Scholar] [CrossRef]
- Gao, L.; Wang, X.; Xie, Z.; Song, W.; Wang, L.; Wu, X.; Qu, F.; Chen, D.; Shen, G. High-performance energy-storage devices based on WO3 nanowire arrays/carbon cloth integrated electrodes. J. Mater. Chem. A 2013, 1, 7167–7173. [Google Scholar] [CrossRef]
- Shinde, P.A.; Lokhande, A.C.; Patil, A.M.; Lokhande, C.D. Facile synthesis of self-assembled WO3 nanorods for high-performance electrochemical capacitor. J. Alloys Compd. 2019, 770, 1130–1137. [Google Scholar] [CrossRef]
- Shinde, P.A.; Lokhande, A.C.; Chodankar, N.R.; Patil, A.M.; Kim, J.H.; Lokhande, C.D. Temperature dependent surface morphological modifications of hexagonal WO3 thin films for high performance supercapacitor application. Electrochim. Acta 2017, 224, 397–404. [Google Scholar] [CrossRef]
- Nayak, A.K.; Das, A.K.; Pradhan, D. High Performance Solid-State Asymmetric Supercapacitor using Green Synthesized Graphene–WO3 Nanowires Nanocomposite. ACS Sustain. Chem. Eng. 2017, 5, 10128–10138. [Google Scholar] [CrossRef]
- Jung, J.; Kim, H. W 18 O 49 nanowires assembled on carbon felt for application to supercapacitors. Appl. Surf. Sci. 2018, 433, 750–755. [Google Scholar] [CrossRef]
- Xu, J.; Ding, T.; Wang, J.; Zhang, J.; Wang, S.; Chen, C.; Fang, Y.; Wu, Z.; Huo, K.; Dai, J. Tungsten Oxide Nanofibers Self-assembled Mesoscopic Microspheres as High-performance Electrodes for Supercapacitor. Electrochim. Acta 2015, 174, 728–734. [Google Scholar] [CrossRef]
- Shao, Z.; Fan, X.; Liu, X.; Yang, Z.; Wang, L.; Chen, Z.; Zhang, W. Hierarchical micro/nanostructured WO3 with structural water for high-performance pseudocapacitors. J. Alloys Compd. 2018, 765, 489–496. [Google Scholar] [CrossRef]
- Gupta, S.P.; Patil, V.B.; Tarwal, N.L.; Bhame, S.D.; Gosavi, S.W.; Mulla, I.S.; Late, D.J.; Suryavanshi, S.S.; Walke, P.S. Enhanced energy density and stability of self-assembled cauliflower of Pd doped monoclinic WO3 nanostructure supercapacitor. Mater. Chem. Phys. 2019, 225, 192–199. [Google Scholar] [CrossRef]
- Zheng, F.; Gong, H.; Li, Z.; Yang, W.; Xu, J.; Hu, P.; Li, Y.; Gong, Y.; Zhen, Q. Tertiary structure of cactus-like WO 3 spheres self-assembled on Cu foil for supercapacitive electrode materials. J. Alloys Compd. 2017, 712, 345–354. [Google Scholar] [CrossRef]
- Ji, S.-H.; Chodankar, N.R.; Kim, D.-H. Aqueous asymmetric supercapacitor based on RuO2-WO3 electrodes. Electrochim. Acta 2019, 325, 134879. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Altomare, M.; Eugénio, S.; Schmuki, P.; Silva, T.M.; Montemor, M.F. On the Supercapacitive Behaviour of Anodic Porous WO3-Based Negative Electrodes. Electrochim. Acta 2017, 232, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Zhou, X.; Xu, L.; Xu, X.; Zhang, L.; Ye, C.; Luo, J.; Chen, W. Hydrothermal preparation and supercapacitive performance of flower-like WO3·H2O/reduced graphene oxide composite. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 609–615. [Google Scholar] [CrossRef]
- Di, J.; Xu, H.; Gai, X.; Yang, R.; Zheng, H. One-step solvothermal synthesis of feather duster-like CNT@WO3 as high-performance electrode for supercapacitor. Mater. Lett. 2019, 246, 129–132. [Google Scholar] [CrossRef]
- Chu, J.; Lu, D.; Wang, X.; Wang, X.; Xiong, S. WO3 nanoflower coated with graphene nanosheet: Synergetic energy storage composite electrode for supercapacitor application. J. Alloys Compd. 2017, 702, 568–572. [Google Scholar] [CrossRef]
- Liu, X.; Sheng, G.; Zhong, M.; Zhou, X. Hybrid nanowires and nanoparticles of WO3 in a carbon aerogel for supercapacitor applications. Nanoscale 2018, 10, 4209–4217. [Google Scholar] [CrossRef]
- Liu, X.; Sheng, G.; Zhong, M.; Zhou, X. Dispersed and size-selected WO3 nanoparticles in carbon aerogel for supercapacitor applications. Mater. Des. 2018, 141, 220–229. [Google Scholar] [CrossRef]
- Shinde, P.A.; Lokhande, V.C.; Patil, A.M.; Ji, T.; Lokhande, C.D. Single-step hydrothermal synthesis of WO3-MnO2 composite as an active material for all-solid-state flexible asymmetric supercapacitor. Int. J. Hydrog. Energy 2018, 43, 2869–2880. [Google Scholar] [CrossRef]
- Yuan, C.; Lin, H.; Lu, H.; Xing, E.; Zhang, Y.; Xie, B. Anodic deposition and capacitive property of nano-WO3·H2O/MnO2 composite as supercapacitor electrode material. Mater. Lett. 2015, 148, 167–170. [Google Scholar] [CrossRef]
- Periasamy, P.; Krishnakumar, T.; Sandhiya, M.; Sathish, M.; Chavali, M.; Siril, P.F.; Devarajan, V. Preparation and comparison of hybridized WO3–V2O5 nanocomposites electrochemical supercapacitor performance in KOH and H2SO4 electrolyte. Mater. Lett. 2019, 236, 702–705. [Google Scholar] [CrossRef]
- Hai, Z.; Akbari, M.K.; Xue, C.; Xu, H.; Solano, E.; Detavernier, C.; Hu, J.; Zhuiykov, S. Atomically-thin WO 3 /TiO 2 heterojunction for supercapacitor electrodes developed by atomic layer deposition. Compos. Commun. 2017, 5, 31–35. [Google Scholar] [CrossRef]
- Hai, Z.; Akbari, M.K.; Wei, Z.; Xue, C.; Xu, H.; Hu, J.; Hyde, L.; Zhuiykov, S. TiO 2 nanoparticles-functionalized two-dimensional WO 3 for high-performance supercapacitors developed by facile two-step ALD process. Mater. Today Commun. 2017, 12, 55–62. [Google Scholar] [CrossRef]
- Tian, J.; Lin, B.; Sun, Y.; Zhang, X.; Yang, H. Porous WO 3 @CuO composites derived from polyoxometalates@metal organic frameworks for supercapacitor. Mater. Lett. 2017, 206, 91–94. [Google Scholar] [CrossRef]
- Zhuzhelskii, D.; Tolstopjatova, E.; Eliseeva, S.; Ivanov, A.; Miao, S.; Kondratiev, V. Electrochemical properties of PEDOT/WO3 composite films for high performance supercapacitor application. Electrochim. Acta 2019, 299, 182–190. [Google Scholar] [CrossRef]
- Das, A.K.; Paria, S.; Maitra, A.; Halder, L.; Bera, A.; Bera, R.; Si, S.K.; De, A.; Ojha, S.; Bera, S.; et al. Highly Rate Capable Nanoflower-like NiSe and WO3@PPy Composite Electrode Materials toward High Energy Density Flexible All-Solid-State Asymmetric Supercapacitor. ACS Appl. Electron. Mater. 2019, 1, 977–990. [Google Scholar] [CrossRef]
- Cong, S.; Tian, Y.; Li, Q.; Zhao, Z.; Geng, F. Single-Crystalline Tungsten Oxide Quantum Dots for Fast Pseudocapacitor and Electrochromic Applications. Adv. Mater. 2014, 26, 4260–4267. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Zhang, G.; Liu, W.; Li, D.; Chen, R.; Zheng, F.; Ni, H. Simple synthesis of 1D, 2D and 3D WO3 nanostructures on stainless steel substrate for high-performance supercapacitors. J. Alloys Compd. 2019, 778, 603–611. [Google Scholar] [CrossRef]
- Yoon, S.; Jo, C.; Noh, S.Y.; Lee, C.W.; Song, J.H.; Lee, J. Development of a high-performance anode for lithium-ion batteries using novel ordered mesoporous tungsten oxide materials with high electrical conductivity. Phys. Chem. Chem. Phys. 2011, 13, 11060–11066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Y.; Xiao, K.; Bedford, N.M.; Lu, X.; Yun, J.; Amal, R.; Wang, D. Refilling Nitrogen to Oxygen Vacancies in Ultrafine Tungsten Oxide Clusters for Superior Lithium Storage. Adv. Energy Mater. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Chavan, H.; Ahmed, A.T.A.; Cho, S.; Kim, J.; Jo, Y.; Pawar, S.M.; Park, Y.; Kim, H.; Im, H. Nanograin tungsten oxide with excess oxygen as a highly reversible anode material for high-performance Li-ion batteries. Mater. Lett. 2018, 215, 233–237. [Google Scholar] [CrossRef]
- Lim, Y.R.; Ko, Y.; Park, J.; Cho, W.I.; Lim, S.A.; Cha, E. Morphology-controlled WO3 and WS2 nanocrystals for improved cycling perfor-mance of lithium ion batteries. J. Electrochem. Sci. Technol. 2019, 10, 89–97. [Google Scholar] [CrossRef]
- Yang, J.; Xu, L.; Yan, S.; Zheng, W. Formation of tungsten trioxide with hierarchical architectures arranged by tiny nanorods for lithium-ion batteries. RSC Adv. 2016, 6, 18071–18076. [Google Scholar] [CrossRef]
- Sasidharan, M.; Gunawardhana, N.; Yoshio, M.; Nakashima, K. WO3 hollow nanospheres for high-lithium storage capacity and good cyclability. Nano Energy 2012, 1, 503–508. [Google Scholar] [CrossRef]
- Zeng, F.; Ren, Y.; Chen, L.; Yang, Y.; Li, Q.; Gu, G. Hierarchical sandwich-type tungsten trioxide nanoplatelets/graphene anode for high-performance lithium-ion batteries with long cycle life. Electrochim. Acta 2016, 190, 964–971. [Google Scholar] [CrossRef]
- Kim, D.-M.; Kim, S.-J.; Lee, Y.-W.; Kwak, D.-H.; Park, H.-C.; Kim, M.-C.; Hwang, B.-M.; Lee, S.; Choi, J.-H.; Hong, S.; et al. Two-dimensional nanocomposites based on tungsten oxide nanoplates and graphene nanosheets for high-performance lithium-ion batteries. Electrochim. Acta 2015, 163, 132–139. [Google Scholar] [CrossRef]
- Gu, X.; Wu, F.; Lei, B.; Wang, J.; Chen, Z.; Xie, K.; Song, Y.; Sun, D.; Sun, L.; Zhou, H.; et al. Three-dimensional nitrogen-doped graphene frameworks anchored with bamboo-like tungsten oxide nanorods as high performance anode materials for lithium ion batteries. J. Power Sources 2016, 320, 231–238. [Google Scholar] [CrossRef]
- Dang, W.; Wang, W.; Yang, Y.; Wang, Y.; Huang, J.; Fang, X.; Wu, L.; Rong, Z.; Chen, X.; Li, X.; et al. One-step hydrothermal synthesis of 2D WO3 nanoplates@ graphene nanocomposite with superior anode performance for lithium-ion battery. Electrochim. Acta 2019, 313, 99–108. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, R.; Wang, M.; Sakamoto, J.; Poudeu, P.F. Hexagonal-WO3 nanorods encapsulated in nitrogen and sulfur co-doped reduced graphene oxide as a high-performance anode material for lithium ion batteries. J. Solid State Chem. 2020, 282, 121068. [Google Scholar] [CrossRef]
- Park, S.K.; Lee, H.J.; Lee, M.H.; Park, H.S. Hierarchically structured reduced graphene oxide/WO3 frameworks for an application into lithium-ion battery anodes. Chem. Eng. J. 2015, 281, 724–729. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Zhou, L.; Liu, Y.; Zhang, W.; Zhao, Z.; Hozzein, W.N.; Alharbi, H.M.S.; Li, W.; Zhao, D. Mesoporous carbon matrix confinement synthesis of ultrasmall WO3 nanocrystals for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 21550–21557. [Google Scholar] [CrossRef]
- Yoon, S.; Woo, S.-G.; Jung, K.-N.; Song, H. Conductive surface modification of cauliflower-like WO3 and its electrochemical properties for lithium-ion batteries. J. Alloys Compd. 2014, 613, 187–192. [Google Scholar] [CrossRef]
- Herdt, T.; Deckenbach, D.; Bruns, M.; Schneider, J.J. Tungsten oxide nanorod architectures as 3D anodes in binder-free lithium-ion batteries. Nanoscale 2018, 11, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, P.; Wan, Q.; Zhang, D.; Volinsky, A.A.; Qu, X. Low-temperature combustion synthesis of hexagonal WO3·0.33H2O@C as anode material for lithium-ion batteries. J. Alloys Compd. 2017, 701, 215–221. [Google Scholar] [CrossRef]
- Bao, K.; Mao, W.; Liu, G.; Ye, L.; Xie, H.; Ji, S.; Wang, D.; Chen, C.; Li, Y. Preparation and electrochemical characterization of ultrathin WO3−x /C nanosheets as anode materials in lithium-ion batteries. Nano Res. 2016, 10, 1903–1911. [Google Scholar] [CrossRef]
- Aguir, K.; Lemire, C.; Lollman, D. Electrical properties of reactively sputtered WO3 thin films as ozone gas sensor. Sens. Actuators B Chem. 2002, 84, 1–5. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, W.; Qin, J.; Zhao, D.; Mao, B.; Xiao, Y.; Cao, M. Oxygen vacancy-rich mesoporous W18O49 nanobelts with ultrahigh initial Coulombic efficiency toward high-performance lithium storage. Electrochim. Acta 2016, 187, 329–339. [Google Scholar] [CrossRef]
- Li, W.; Sasaki, A.; Oozu, H.; Aoki, K.; Kakushima, K.; Kataoka, Y.; Nishiyama, A.; Sugii, N.; Wakabayashi, H.; Tsutsui, K.; et al. Improvement of charge/discharge performance for lithium-ion batteries with tungsten trioxide electrodes. Microelectron. Reliab. 2015, 55, 402–406. [Google Scholar] [CrossRef]
- Pumera, M. Graphene-based nanomaterials for energy storage. Energy Environ. Sci. 2010, 4, 668–674. [Google Scholar] [CrossRef]
- Ghosh, T.; Oh, W. Review on Reduced Graphene Oxide by Chemical Exfoliation Method and its Simpler Alternative of Ultrasonication and Heat Treatment Method for Obtaining Graphene. J. Photocatal. Sci. 2012, 3, 17–23. [Google Scholar]
- Liang, C.; Li, Z.; Dai, S. Mesoporous Carbon Materials: Synthesis and Modification. Angew. Chem. Int. Ed. 2008, 47, 3696–3717. [Google Scholar] [CrossRef]
- Kim, H.-S.; Ryu, J.H.; Kim, J.; Hwang, K.; Kang, H.; Oh, S.M.; Son, H.; Yoon, S. Ordered mesoporous tungsten oxide–carbon nanocomposite for use as a highly reversible negative electrode in lithium-ion batteries. J. Alloys Compd. 2020, 832, 154816. [Google Scholar] [CrossRef]
- Khoo, E.; Lee, P.S.; Ma, J. Electrophoretic deposition (EPD) of WO3 nanorods for electrochromic application. J. Eur. Ceram. Soc. 2010, 30, 1139–1144. [Google Scholar] [CrossRef]
- Kondalkar, V.V.; Kharade, R.R.; Mali, S.S.; Mane, R.; Patil, P.; Choudhury, S.; Bhosale, P. Nanobrick-like WO3 thin films: Hydrothermal synthesis and electrochromic application. Superlattices Microstruct. 2014, 73, 290–295. [Google Scholar] [CrossRef]
- Bhosale, N.Y.; Mali, S.S.; Hong, C.K.; Kadam, A.V. Hydrothermal synthesis of WO3 nanoflowers on etched ITO and their electrochromic properties. Electrochim. Acta 2017, 246, 1112–1120. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, Q.; Wei, W.; Chen, Z.; Zhu, Y.; Zhang, P.; Zhang, Z.; Gao, Y. WO3 quantum-dots electrochromism. Nano Energy 2020, 68, 104350. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, J.-P.; Xia, X.-H.; Wang, X.-L.; Gu, C.-D. Hydrothermally synthesized WO3 nanowire arrays with highly improved electrochromic performance. J. Mater. Chem. 2011, 21, 5492–5498. [Google Scholar] [CrossRef]
- Zhou, J.; Wei, Y.; Luo, G.; Zheng, J.; Xu, C. Electrochromic properties of vertically aligned Ni-doped WO3 nanostructure films and their application in complementary electrochromic devices. J. Mater. Chem. C 2016, 4, 1613–1622. [Google Scholar] [CrossRef]
- Chang, X.; Sun, S.; Li, Z.; Xu, X.; Qiu, Y. Assembly of tungsten oxide nanobundles and their electrochromic properties. Appl. Surf. Sci. 2011, 257, 5726–5730. [Google Scholar] [CrossRef]
- Azam, A.; Kim, J.; Park, J.; Novak, T.G.; Tiwari, A.P.; Song, S.H.; Kim, B.; Jeon, S. Two-Dimensional WO3 Nanosheets Chemically Converted from Layered WS2 for High-Performance Electrochromic Devices. Nano Lett. 2018, 18, 5646–5651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Xia, X.; Gu, C.; Tu, J. Electrochromic behavior of WO3 nanotree films prepared by hydrothermal oxidation. Sol. Energy Mater. Sol. Cells 2011, 95, 2107–2112. [Google Scholar] [CrossRef]
- Li, Y.; McMaster, W.A.; Wei, H.; Chen, D.; Caruso, R.A. Enhanced Electrochromic Properties of WO3Nanotree-like Structures Synthesized via a Two-Step Solvothermal Process Showing Promise for Electrochromic Window Application. ACS Appl. Nano Mater. 2018, 1, 2552–2558. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, J.; Ke, L.; Liu, X.; Demir, H.V.; Yang, M.F.; Sun, X.W. Electrochromic properties of nanostructured tungsten trioxide (hydrate) films and their applications in a complementary electrochromic device. Electrochim. Acta 2012, 63, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Peng, M.-D.; Zhang, Y.-Z.; Song, L.-X.; Wu, L.-N.; Hu, X.-F.; Zhang, Y.-L. Electrochemical stability properties of titanium-doped WO3 electrochromic thin films. Surf. Eng. 2016, 33, 305–309. [Google Scholar] [CrossRef]
- Koo, B.-R.; Kim, K.-H.; Ahn, H.-J. Switching electrochromic performance improvement enabled by highly developed mesopores and oxygen vacancy defects of Fe-doped WO3 films. Appl. Surf. Sci. 2018, 453, 238–244. [Google Scholar] [CrossRef]
- Lee, C.-T.; Chiang, D.; Chiu, P.-K.; Chang, C.-M.; Jaing, C.-C.; Ou, S.-L.; Kao, K.-S. WO3 Electrochromic Thin Films Doped with Carbon. IEEE Trans. Magn. 2014, 50, 1–4. [Google Scholar] [CrossRef]
- Atak, G.; Pehlivan, I.B.; Montero, J.; Primetzhofer, D.; Granqvist, C.G.; Niklasson, G.A. Electrochromism of nitrogen-doped tungsten oxide thin films. Mater. Today Proc. 2020, 33, 2434–2439. [Google Scholar] [CrossRef]
- Bon-Ryul, K.; Kim, K.-H.; Ahn, H.-J. Novel tunneled phosphorus-doped WO3 films achieved using ignited red phosphorus for stable and fast switching electrochromic performances. Nanoscale 2019, 11, 3318–3325. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, M.; Zhang, Y. Optical and electrochemical properties of Ni doped WO 3 -MoO 3 films prepared by sol-gel process. Proc. SPIE 2007, 6722, 1–5. [Google Scholar] [CrossRef]
- De León, J.O.-R.; Acosta, D.; Pal, U.; Castañeda, L. Improving electrochromic behavior of spray pyrolised WO3 thin solid films by Mo doping. Electrochim. Acta 2011, 56, 2599–2605. [Google Scholar] [CrossRef]
- Madhavi, V.; Kumar, P.J.; Kondaiah, P.; Hussain, O.M.; Uthanna, S. Effect of molybdenum doping on the electrochromic properties of tungsten oxide thin films by RF magnetron sputtering. Ionics 2014, 20, 1737–1745. [Google Scholar] [CrossRef]
- Shen, K.; Sheng, K.; Wang, Z.; Zheng, J.; Xu, C. Cobalt ions doped tungsten oxide nanowires achieved vertically aligned nanostructure with enhanced electrochromic properties. Appl. Surf. Sci. 2020, 501, 144003. [Google Scholar] [CrossRef]
- Mukherjee, R.; Sahay, P. Improved electrochromic performance in sprayed WO 3 thin films upon Sb doping. J. Alloys Compd. 2016, 660, 336–341. [Google Scholar] [CrossRef]
- Najafi-Ashtiani, H.; Bahari, A.; Gholipour, S. Investigation of coloration efficiency for tungsten oxide–silver nanocomposite thin films with different surface morphologies. J. Mater. Sci. Mater. Electron. 2018, 29, 5820–5829. [Google Scholar] [CrossRef]
- Alsawafta, M.; Golestani, Y.M.; Phonemac, T.; Badilescu, S.; Stancovski, V.; Truong, V.-V. Electrochromic Properties of Sol-Gel Synthesized Macroporous Tungsten Oxide Films Doped with Gold Nanoparticles. J. Electrochem. Soc. 2014, 161, 276–283. [Google Scholar] [CrossRef]
- Yin, Y.; Lan, C.; Hu, S.; Li, C. Effect of Gd-doping on electrochromic properties of sputter deposited WO3 films. J. Alloys Compd. 2018, 739, 623–631. [Google Scholar] [CrossRef]
- Shen, L.; Zheng, J.; Xu, C. Enhanced electrochromic switches and tunable green fluorescence based on terbium ion doped WO3 films. Nanoscale 2019, 11, 23049–23057. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, K.; Zhang, X.; Yuan, W.; Ning, H.; Tao, R.; Liu, X.; Yao, R.; Peng, J. Enhanced Transmittance Modulation of SiO2-Doped Crystalline WO3 Films Prepared from a Polyethylene Oxide (PEO) Template. Coatings 2018, 8, 228. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Ko, K.-W.; Sarwar, S.; Lee, M.-S.; Park, S.; Hong, S.; Han, C.-H. Enhanced electrochromic properties of TiO2 nanocrystal embedded amorphous WO3 films. Electrochim. Acta 2018, 278, 396–404. [Google Scholar] [CrossRef]
- Meenakshi, M.; Gowthami, V.; Perumal, P.; Sivakumar, R.; Sanjeeviraja, C. Influence of Dopant Concentration on the Electrochromic Properties of Tungsten Oxide Thin Films. Electrochim. Acta 2015, 174, 302–314. [Google Scholar] [CrossRef]
- Ding, J.; Liu, Z.; Wei, A.; Chen, T.; Zhang, H. Study of electrochromic characteristics in the near-infrared region of electrochromic devices based on solution-processed amorphous WO3 films. Mater. Sci. Semicond. Process. 2018, 88, 73–78. [Google Scholar] [CrossRef]
- Chang-Jian, C.-W.; Cho, E.-C.; Yen, S.-C.; Ho, B.-C.; Lee, K.-C.; Huang, J.-H.; Hsiao, Y.-S. Facile preparation of WO 3 /PEDOT:PSS composite for inkjet printed electrochromic window and its performance for heat shielding. Dye. Pigment. 2018, 148, 465–473. [Google Scholar] [CrossRef]
- Li, G.; Wu, G.; Guo, C.; Wang, B. Fabrication of one-dimensional W18O49 nanomaterial for the near infrared shielding. Mater. Lett. 2016, 169, 227–230. [Google Scholar] [CrossRef]
- Yang, L.; Ge, D.; Zhao, J.; Ding, Y.; Kong, X.; Li, Y. Improved electrochromic performance of ordered macroporous tungsten oxide films for IR electrochromic device. Sol. Energy Mater. Sol. Cells 2012, 100, 251–257. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Yeo, L.P.; Kei, T.C.; Mandler, D.; Magdassi, S.; Tok, A.I.Y. Efficient Near Infrared Modulation with High Visible Transparency Using SnO 2 –WO 3 Nanostructure for Advanced Smart Windows. Adv. Opt. Mater. 2019, 7, 1–10. [Google Scholar] [CrossRef]
- Ling, H.; Yeo, L.P.; Wang, Z.; Li, X.; Mandler, D.; Magdassi, S.; Tok, A.I.Y. TiO2–WO3 core–shell inverse opal structure with enhanced electrochromic performance in NIR region. J. Mater. Chem. C 2018, 6, 8488–8494. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, S.; Zhang, T.; Yao, Q.; Fisher, A.C.; Lee, J.Y. Monoclinic oxygen-deficient tungsten oxide nanowires for dynamic and independent control of near-infrared and visible light transmittance. Mater. Horiz. 2018, 5, 291–297. [Google Scholar] [CrossRef]
- Costa, C.; Pinheiro, C.; Henriques, I.; Laia, C.A.T. Inkjet Printing of Sol–Gel Synthesized Hydrated Tungsten Oxide Nanoparticles for Flexible Electrochromic Devices. ACS Appl. Mater. Interfaces 2012, 4, 1330–1340. [Google Scholar] [CrossRef]
- Pan, J.; Zheng, R.; Wang, Y.; Ye, X.; Wan, Z.; Jia, C.; Weng, X.; Xie, J.; Deng, L. A high-performance electrochromic device assembled with hexagonal WO3 and NiO/PB composite nanosheet electrodes towards energy storage smart window. Sol. Energy Mater. Sol. Cells 2020, 207, 110337. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, X.; Li, Z.; Wang, B.; Wang, D.; Nie, G. High Performance Multicolor Intelligent Supercapacitor and Its Quantitative Monitoring of Energy Storage Level by Electrochromic Parameters. ACS Appl. Energy Mater. 2020, 3, 2727–2736. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, Y.; Li, W.; Dou, S.; Wang, L.; Qu, H.; Zhao, J.; Li, Y. Preparation and performances of all-solid-state variable infrared emittance devices based on amorphous and crystalline WO3 electrochromic thin film. Sol. Energy Mater. Sol. Cells 2019, 200, 109916. [Google Scholar] [CrossRef]
- Cho, S.I.; Lee, S.B. Fast Electrochemistry of Conductive Polymer Nanotubes: Synthesis, Mechanism, and Application. Accounts Chem. Res. 2008, 41, 699–707. [Google Scholar] [CrossRef]
- Wang, W.-Q.; Wang, X.-L.; Xia, X.-H.; Yao, Z.-J.; Zhong, Y.; Tu, J.-P. Enhanced electrochromic and energy storage performance in mesoporous WO3 film and its application in a bi-functional smart window. Nanoscale 2018, 10, 8162–8169. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Bi, Z.; Chen, Y.; He, X.; Guo, X.; Gao, X.; Li, X. Electrodeposited Mo-doped WO3 film with large optical modulation and high areal capacitance toward electrochromic energy-storage applications. Appl. Surf. Sci. 2018, 459, 774–781. [Google Scholar] [CrossRef]
- Cai, G.; Wang, X.; Cui, M.; Darmawan, P.; Wang, J.; Eh, A.L.-S.; Lee, P.S. Electrochromo-supercapacitor based on direct growth of NiO nanoparticles. Nano Energy 2015, 12, 258–267. [Google Scholar] [CrossRef]
- Wei, D.; Scherer, M.R.J.; Bower, C.; Andrew, P.; Ryhänen, T.; Steiner, U. A Nanostructured Electrochromic Supercapacitor. Nano Lett. 2012, 12, 1857–1862. [Google Scholar] [CrossRef]
- Wang, K.; Wu, H.; Meng, Y.; Zhang, Y.; Wei, Z. Integrated energy storage and electrochromic function in one flexible device: An energy storage smart window. Energy Environ. Sci. 2012, 5, 8384–8389. [Google Scholar] [CrossRef]
- Xia, X.; Ku, Z.; Zhou, D.; Zhong, Y.; Zhang, Y.; Wang, Y.; Huang, M.J.; Tu, J.; Fan, H.J. Perovskite solar cell powered electrochromic batteries for smart windows. Mater. Horiz. 2016, 3, 588–595. [Google Scholar] [CrossRef]
- He, X.; Li, X.; Bi, Z.; Chen, Y.; Xu, X.; Gao, X. Dual-functional electrochromic and energy-storage electrodes based on tungsten trioxide nanostructures. J. Solid State Electrochem. 2018, 22, 2579–2586. [Google Scholar] [CrossRef]
- Bi, Z.; Li, X.; He, X.; Chen, Y.; Xu, X.; Gao, X. Integrated electrochromism and energy storage applications based on tungsten trioxide monohydrate nanosheets by novel one-step low temperature synthesis. Sol. Energy Mater. Sol. Cells 2018, 183, 59–65. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Kim, J.; Jo, Y.; Woo, H.; Cho, S.; Pawar, S.M.; Lee, S.; Gunjakar, J.L.; Cho, Y.; Hou, B.; et al. Highly efficient electro-optically tunable smart-supercapacitors using an oxygen-excess nanograin tungsten oxide thin film. Sol. Energy Mater. Sol. Cells 2017, 166, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yao, Z.; Wang, X.; Xia, X.; Gu, C.; Tu, J. Niobium doped tungsten oxide mesoporous film with enhanced electrochromic and electrochemical energy storage properties. J. Colloid Interface Sci. 2019, 535, 300–307. [Google Scholar] [CrossRef]
- Zhou, D.; Shi, F.; Xie, D.; Wang, D.; Xia, X.; Wang, X.; Gu, C.; Tu, J. Bi-functional Mo-doped WO 3 nanowire array electrochromism-plus electrochemical energy storage. J. Colloid Interface Sci. 2016, 465, 112–120. [Google Scholar] [CrossRef]
- Wei, H.; Yan, X.; Wu, S.; Luo, Z.; Wei, S.; Guo, Z. Electropolymerized Polyaniline Stabilized Tungsten Oxide Nanocomposite Films: Electrochromic Behavior and Electrochemical Energy Storage. J. Phys. Chem. C 2012, 116, 25052–25064. [Google Scholar] [CrossRef]
- Nwanya, A.C.; Jafta, C.J.; Ejikeme, P.M.; Ugwuoke, P.E.; Reddy, M.; Osuji, R.U.; Ozoemena, K.I.; Ezema, F.I. Electrochromic and electrochemical capacitive properties of tungsten oxide and its polyaniline nanocomposite films obtained by chemical bath deposition method. Electrochim. Acta 2014, 128, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Li, N.; Wang, Y.; Ma, X.; Zhao, J.; Qiang, L.; Hou, S.; Ji, J.; Li, Y. Bifunctional urchin-like WO3@PANI electrodes for superior electrochromic behavior and lithium-ion battery. J. Mater. Sci. Mater. Electron. 2018, 29, 14803–14812. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, X.; Li, Z.; Wang, D.; Nie, G. A novel solid-state electrochromic supercapacitor with high energy storage capacity and cycle stability based on poly(5-formylindole)/WO3 honeycombed porous nanocomposites. Chem. Eng. J. 2020, 384, 123370. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, J.-P.; Zhang, D.; Qiao, Y.-Q.; Xia, X.-H.; Wang, X.-L.; Gu, C.-D. Multicolor electrochromic polyaniline–WO3 hybrid thin films: One-pot molecular assembling synthesis. J. Mater. Chem. 2011, 21, 17316–17324. [Google Scholar] [CrossRef]
- Cai, G.; Darmawan, P.; Cui, M.; Wang, J.; Chen, J.; Magdassi, S.; Lee, P.S. Highly Stable Transparent Conductive Silver Grid/PEDOT:PSS Electrodes for Integrated Bifunctional Flexible Electrochromic Supercapacitors. Adv. Energy Mater. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Sun, P.; Liu, Y.; Qiu, M.; Tong, Y.; Mai, W. In Situ Monitoring Small Energy Storage Change of Electrochromic Supercapacitors via Perovskite Photodetectors. Small Methods 2020, 4, 1–6. [Google Scholar] [CrossRef]
- Yang, P.; Sun, P.; Mai, W. Electrochromic energy storage devices. Mater. Today 2016, 19, 394–402. [Google Scholar] [CrossRef]
- Cai, G.; Wang, J.; Lee, P.S. Next-Generation Multifunctional Electrochromic Devices. Acc. Chem. Res. 2016, 49, 1469–1476. [Google Scholar] [CrossRef]
- Cai, G.; Darmawan, P.; Cheng, X.; Lee, P.S. Inkjet Printed Large Area Multifunctional Smart Windows. Adv. Energy Mater. 2017, 7, 1602598. [Google Scholar] [CrossRef]
- Deb, S.K. Opportunities and challenges in science and technology of WO3 for electrochromic and related applications. Sol. Energy Mater. Sol. Cells 2008, 92, 245–258. [Google Scholar] [CrossRef]
- Bi, Z.; Li, X.; Chen, Y.; Xu, X.; Zhang, S.; Zhu, Q. Bi-functional flexible electrodes based on tungsten trioxide/zinc oxide nanocomposites for electrochromic and energy storage applications. Electrochim. Acta 2017, 227, 61–68. [Google Scholar] [CrossRef]
- Xu, Z.; Li, W.; Huang, J.; Guo, X.; Liu, Q.; Yu, R.; Miao, W.; Zhou, B.; Guo, W.; Liu, X. Flexible, controllable and angle-independent photoelectrochromic display enabled by smart sunlight management. Nano Energy 2019, 63, 103830. [Google Scholar] [CrossRef]
- Wang, Z.; Chiu, H.; Paolella, A.; Zaghib, K.; Demopoulos, G.P. Lithium Photo-intercalation of CdS-Sensitized WO 3 Anode for Energy Storage and Photoelectrochromic Applications. ChemSusChem 2019, 12, 2220–2230. [Google Scholar] [CrossRef]
- Xie, Z.; Jin, X.; Chen, G.; Xu, J.; Chen, D.; Shen, G. Integrated smart electrochromic windows for energy saving and storage applications. Chem. Commun. 2014, 50, 608–610. [Google Scholar] [CrossRef]
- Chang, X.; Hu, R.; Sun, S.; Liu, J.; Lei, Y.; Liu, T.; Dong, L.; Yin, Y. Sunlight-charged electrochromic battery based on hybrid film of tungsten oxide and polyaniline. Appl. Surf. Sci. 2018, 441, 105–112. [Google Scholar] [CrossRef]
- Yun, T.G.; Kim, D.; Kim, Y.H.; Park, M.; Hyun, S.; Han, S.M. Photoresponsive Smart Coloration Electrochromic Supercapacitor. Adv. Mater. 2017, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Jiao, X.; Wang, T.; Chen, D. The fast and reversible intrinsic photochromic response of hydrated tungsten oxide nanosheets. J. Mater. Chem. C 2015, 3, 7597–7603. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, R.; Lu, Y.; Wu, Y.; Yuan, J.; Liu, J. Highly Efficient Photochromic Tungsten Oxide@PNIPAM Composite Spheres with a Fast Response. ACS Appl. Mater. Interfaces 2021, 13, 4220–4229. [Google Scholar] [CrossRef]
- Wang, S.; Fan, W.; Liu, Z.; Yu, A.; Jiang, X. Advances on tungsten oxide based photochromic materials: Strategies to improve their photochromic properties. J. Mater. Chem. C 2018, 6, 191–212. [Google Scholar] [CrossRef]
- Bignozzi, C.A.; Caramori, S.; Cristino, V.; Argazzi, R.; Meda, L.; Tacca, A. Nanostructured photoelectrodes based on WO3: Applications to photooxidation of aqueous electrolytes. Chem. Soc. Rev. 2013, 42, 2228–2246. [Google Scholar] [CrossRef]
- Jin, J.; Yu, J.; Guo, D.; Cui, C.; Ho, W. A Hierarchical Z-Scheme CdS-WO3Photocatalyst with Enhanced CO2 Reduction Activity. Small 2015, 11, 5262–5271. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Fleisch, M.; Bahnemann, D.W. Surface-grafted WO3/TiO2 photocatalysts: Enhanced visible-light activity towards indoor air purification. Catal. Today 2018, 313, 63–71. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Palmas, S.; Castresana, P.A.; Mais, L.; Vacca, A.; Mascia, M.; Ricci, P.C. TiO2–WO3 nanostructured systems for photoelectrochemical applications. RSC Adv. 2016, 6, 101671–101682. [Google Scholar] [CrossRef] [Green Version]
- Castro, I.; Byzynski, G.; Dawson, M.; Ribeiro, C. Charge transfer mechanism of WO 3 /TiO 2 heterostructure for photoelectrochemical water splitting. J. Photochem. Photobiol. A Chem. 2017, 339, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhou, X.; Luo, W.; Cheng, X.; Zhu, Y.; El-Toni, A.M.; Khan, A.; Deng, Y.; Zhao, D. Pore Engineering of Mesoporous Tungsten Oxides for Ultrasensitive Gas Sensing. Adv. Mater. Interfaces 2018, 6, 1–9. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Xu, Y.; Li, Y. Nanosheet-assembled hierarchical WO3 flower-like nanostructures: Hydrothermal synthesis and NH3-sensing properties. Mater. Lett. 2019, 250, 155–158. [Google Scholar] [CrossRef]
- Evecan, D.; Zayim, E. Özkan Highly uniform electrochromic tungsten oxide thin films deposited by e-beam evaporation for energy saving systems. Curr. Appl. Phys. 2019, 19, 198–203. [Google Scholar] [CrossRef]
- Wang, C.; Sun, R.; Li, X.; Sun, Y.; Sun, P.; Liu, F.; Lu, G. Hierarchical flower-like WO3 nanostructures and their gas sensing properties. Sens. Actuators B Chem. 2014, 204, 224–230. [Google Scholar] [CrossRef]
- Zeng, W.; Li, Y.; Miao, B.; Pan, K. Hydrothermal synthesis and gas sensing properties of WO3H2O with different morphologies. Phys. E Low-Dimens. Syst. Nano Struct. 2014, 56, 183–188. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, Y.; Zou, C.; Xu, K.; Luo, X.; Luo, T.; Li, J.; Yang, Q.; Shi, P.; Yuan, C. 2D ultra-thin WO3 nanosheets with dominant {002} crystal facets for high-performance xylene sensing and methyl orange photocatalytic degradation. J. Alloys Compd. 2019, 783, 848–854. [Google Scholar] [CrossRef]


















| Products and Structures | Synthesis Method | Electrochemical Performances | |||
|---|---|---|---|---|---|
| Potential Window, Reference Electrode, Electrolyte | Maximum Specific Capacity | Cycling Condition, Cycles, Capacity Retained | |||
| Single phase WO3 nanostructures | WO3 nanofibers [67] | Hydrothermal | −0.65–0 V vs. Ag/AgCl, H+ | 2 mA cm−2, 1.72 F cm−2 | 10 mV s−1, 6000 cycles, 79.1% |
| WO3-x nanorods [68] | Hydrothermal + annealing in hydrogen atmosphere | −10 V vs. SCE, 5 M LiCl | 1 mA cm−2, 1.83 F cm−2 | ----, 10,000 cycles, 74.8% | |
| WO3 nanosheets [69] | Alcohol-thermal process | −1.0–0.5 V vs. Ag/AgCl, 0.5 M Na2SO4 | 5 mA cm−2, 0.659 F cm−2 | ---, 10,000 cycles, almost no decrease | |
| WO3 nanotubes [70] | Hydrothermal | −0.65–0.05 V vs. Ag/AgCl, 0.5 M H2SO4 | 3 mA cm−2, 2.58 F cm−2 1 A g−1, 615.7 F g−1 | 2.5 A g−1, 6000 cycles, 85.11% (decreased from 496.4 to 422.5 F g−1) | |
| Furball-like WO3 microspheres [50] | Hydrothermal | −0.3–0.4 V vs. SCE, 2 M H2SO4 | 2 mA cm−2, 8.35 F cm−2 (=708.0 F g−1) | 2 mA cm−2, 10,000 cycles, 93.4% | |
| WO3 nanorods array [71] | Hydrothermal | −0.6–0.3 V vs. Ag/AgCl, 2 M H2SO4 | 10 A cm−2, 5.21 F cm−2 1 A g−1, 521 F g−1 | 3 A g−1, 2000 cycles, nearly 100% | |
| h-WO3 nanorods [72] | Hydrothermal | −0.7–0.2 V vs. SCE, 1 M H2SO4 | 5 mV s−1, 538 F g−1 | 100 mV s−1, 2000 CV cycles, 85% | |
| h-WO3 nanorods [73] | Hydrothermal | −0.5–0 V vs. SCE, 1 M H2SO4 | 0.35 A g−1, 694 F g−1; 0.93 A g−1, 484 F g−1 | 50 mV s−1, 2000 cycles, 87% | |
| WO3 Nanowires [74] | Solvothermal | −0.4–0.6 V vs. SCE, 0.1 M H2SO4 | 1 A g−1, 465 F g−1 | ----, 2000 cycles, 97.7% | |
| W18O49 Nanowires [75] | Solvothermal | −0.4–0.4 V vs. SCE, 1 M H2SO4 | 1 A g−1, 588.33 F g−1 | 1 A g−1, 5000 cycles, 88% | |
| h-WO3 nanoflake arrays [51] | Hydrothermal | 1.0–1.8 V vs. Ag/AgCl, 1 M Na2SO4 | 0.5 A g−1, 538 F g−1 | ----, 5000 cycles, 95.5% | |
| WO3 nanospheres [76] | Hydrothermal | SCE, 2 M H2SO4 | 0.5 A g−1, 797.05 F g−1 | 5 A g−1, 2000 cycles, 100.47% | |
| Frisbee-like h-WO3*0.28H2O [77] | Hydrothermal | −0.6–0.3 V vs. Ag/AgCl, 1 M H2SO4 | 0.5 A g−1, 391 F g−1 | 10 A g−1, 2000 cycles, 100% | |
| 3% Pd-doped WO3 nanobricks [78] | Hydrothermal | −0.7–0.1 V vs. Ag/AgCl, 1 M Na2SO4 | 0.5 A g−1, 33.34 F g−1 | 1 A g−1, 1100 cycles, 86.95% | |
| Cactus-like WO3 microspheres [79] | Hydrothermal | 0.0–0.6 V vs. Ag/AgCl, 1 M Na2SO4 | 0.5 A g−1, 485 F g−1 | 1 A g−1, 5000 cycles, 93% | |
| Cactus-like WO3 microspheres [80] | Hydrothermal | −0.6–0.2 V vs. SCE, 2 M H2SO4 | 5 mV s−1, 970.26 F g−1 | ----, ----, ---- | |
| Pancake-like h-WO3 [52] | Hydrothermal | −0.3–0.2 V vs. Ag/AgCl, 0.5 M H2SO4 | 0.37 A g−1, 605.5 F g−1; 7.5 A g−1, 276.0 F g−1 | 50 mV s−1, 4000 cycles, 110.2% | |
| WO3 nanochannels [81] | Electrochemical anodization | −0.8–0.5 V, 1 M Na2SO4 | 2 A cm−3, 397 F cm−3 | 10 A cm−3, 3500 cycles, 114% | |
| WO3-carbon composites | Flower-like hierarchical WO3·H2O/reduced graphene oxide (rGO) [82] | Hydrothermal | −0.4–0.1 V vs. SCE, 1 M H2SO4 | 1 A g−1, 244 F g−1; 10 A g−1, 78 F g−1 | 4 A g−1, 900 cycles, 97% |
| Feather duster-like carbon nanotube (CNT)@WO3 [83] | One-step solvothermal | −1–−0.3 V vs. Hg/HgSO4, 0.5 M H2SO4 | 0.5 A g−1 496 F g−1; 10 A g−1, 407 F g−1 | 100 mV s−1, 8000 cycles, 196.3% | |
| Multi-walled carbon nanotubes-tungsten trioxide [49] | Hydrothermal | −0.6–0 V vs. SCE, 1 M LiClO4 | 2 mA cm−2, 429.6 F g−1 (1.55 F cm−2) | 100 mV s−1, 5000 cycles, 94.3% | |
| WO3-rGO nanoflowers [84] | Hydrothermal | −0.4–0.3 V, 0.5 M H2SO4 | 1 A g−1, 495 F g−1 | 1 A g−1, 1000 cycles, 87.5% | |
| WO3 nanoparticles and nanowires in carbon aerogel [85] | ---- | −0.3–0.5 V vs. Ag/AgCl, 2 M H2SO4 | 5 mV s−1, 609 F g−1 | 50 mV s−1, 1000 cycles, 98% | |
| WO3 nanoparticles in carbon aerogel [86] | Solvent immersion + calcination | −0.3–0.5 V vs. Ag/AgCl, 2 M H2SO4 | 5 mV s−1, 1055 F g−1 | 500 mV s−1, 3000 cycles, 96% 50 mV s−1, 1000 cycling, 101% | |
| WO3-transition oxide composites | Binder-free and additive-less WO3-MnO2 [87] | Hydrothermal | −0.6–0.6 V vs. SCE, 1 M Na2SO4 | 5 mV s−1, 609 F g−1 2 mA cm−2, 540 F g−1 | 100 mV s−1, 2000 cycles, 89% |
| WO3*H2O/MnO2 nanosheets [88] | Anodic deposition | −0.1–0.9 V vs. SCE, 0.5 M Na2SO4 | 0.5 A g−1, 363 F g−1 | 2 A g−1, 5000 cycles, 93.8% | |
| WO3–V2O5 nanocomposites [89] | Microwave assisted wet chemical route | KOH electrolyte | ----, 173 F g−1 | ----, 5000 cycles, 126% | |
| 2D WO3/TiO2 heterojunction [90] | Atomic layer deposition (ALD) | 0.0–0.8 V vs. Ag/AgCl, 1 M H2SO4 | 1 A g−1, 625.53 F g−1 | 6 A g−1, 2000 cycles, 97.98% | |
| TiO2 nanoparticles-functionalized 2D WO3 film [91] | Two-step atomic layer deposition process + post-annealing | 0.0–0.8 V vs. Ag/AgCl, 1 M H2SO4 | 1.5 A g−1, 342.5 F g−1 30 A g−1, 285.3 F g−1 | 6 A g−1, 2000 cycles, 94.7% | |
| Porous WO3@CuO [92] | Template assisted method | 0.0–0.5 V vs. SCE, 6 M KOH | 1 A g−1, 284 F g−1 | ----, 1500 cycles, 85.2% | |
| WO3-organic materials composites | PEDOT/WO3 [93] | Electrochemical deposition | −0.3–0.0 V vs. Ag/AgCl, (in 3 M NaCl), 0.5 M H2SO4 | 1.4 A g−1, 615 F g−1 10 A g−1, 308 F g−1 | ----, ----,---- |
| WO3@PPy [94] | In situ oxidative polymerization process | −0.8–0.0 V vs. SCE, 2 M KOH | 2 A g−1, 586 F g−1; 20 A g−1, 78% retained | 5000 cycles, no significant changes in resistive property and morphology | |
| Products and Structures | Synthesis Method | Electrochemical Performances | |||
|---|---|---|---|---|---|
| Initial Efficiency | Voltage Window, Current Density, Capacity (Initial/Second) | Current Density/(mA/g), Cycles, Capacity Retained | |||
| Non-stochiometric tungsten oxides | m-WO3-x [97] | Template method | 53% | 0–2.5 V, ---,748 mA h g−1 (1st) | ---,---, --- |
| N-WOx [98] | Thermal annealing | 52.2% | 0–3.0 V, 100 mA g−1, 1760 mA h g−1 (1st); 817 mA h g−1 (2nd) | 100 mA/g, 150 cycles, 954 mA h g−1 10 A g−1, 4000 cycles, 228 mA h g−1 | |
| Nanogranular WO3 with excess oxygen [99] | Magnetron sputtering | --- | 0–3.0 V, 100 mA g−1, 778.8 mA h g−1 (1st) | 1 A g−1, 500 cycles, 217% retained | |
| Nanostructured tungsten oxides | WO3 Nanotubes [70] | Hydrothermal | 77.8% | 0–3.0 V, 100 mA g−1, 1121.4 mA h g−1 (1st) | 100 mA g−1, 200 cycles, 900 mA h g−1 |
| WO3 nanowires [100] | Hydrothermal | 55.3% | 0–3.0 V, 0.1 C, 954 mA h g−1 (1st) | 0.1 C, 100 cycles, 552 mA h g−1 | |
| Flower-like h-WO3 [101] | Hydrothermal + calcination | --- | 0–3.0 V, 100 mA g−1, 2086.4 mA h g−1 (1st) | 100 mA g−1, 100 cycles, 720.5 mA h g−1 | |
| WO3 hollow nanospheres [102] | Soft template assisted method | 74.0% | 0–3.0 V, 0.2 C, 1054 mA h g−1 (1st) | 0.2 C, 100 cycles, 294 mA h g−1 | |
| Carbon-tungsten oxides composites | 3D sandwich-type architecture with 2D WO3 nanoplatelets and 2D GS [103] | Hydrothermal + ultrasonic stirring + thermal treatment | 71.8% | 0–3.0 V, 72 mA g−1, 1262 mA h g−1 (1st) | 1800 mA g−1, 500 cycles, 397 mA h g−1 |
| WO3 nanoplates and graphene nanosheets 2D nanocomposites [104] | Hydrothermal + heating process | --- | ---, ---, --- | 400 mA g−1, 50 cycles, 455 mA h g−1 (64.3% retained) | |
| Bamboo-like WO3 nanorods anchored on 3D nitrogen-doped graphene frameworks [105] | Hydrothermal + heating process | 64.5% | 0–3.0 V, 1280 mA h g−1 (1st) | 80 mA/g, 100 cycles, 828 mA h g−1 (73.8% retained) | |
| WO3 nanosheet@rGO square particles [106] | Hydrothermal | 87.9% | 0–3.0 V, 100 mA g−1, 1143 mA h g−1 (1st) | 100 mA g−1, 150 cycles, 1005.7 mA h/g | |
| h-WO3 nanorods embedded into nitrogen, sulfur co-doped rGO nanosheets (54 wt %) [107] | Ultrasonic processing + hydrothermal | --- | 0–3.0 V, 100 mA g−1, 1030 mA h g−1 (1st), 816.3 mA h g−1 (2nd) | 1500 mA g−1, 200 cycles, 196 mA h g−1 | |
| WO3 particles deposited on 3D macroporous rGO frameworks [108] | Hydrothermal + freeze-drying | 57.23% | 0–3.0 V, 50 mA g−1, 1120 mA h g−1 (1st), 719 mA h g−1 (2nd) | 150 mA g−1, 100 cycles, 487 mA h g−1 (~99% retained) | |
| Ordered mesoporous carbon/WO3 [109] | Evaporation induced self-assembly | 56.2% | 0–3.0 V, 100 A g−1, 1275 mA h g−1 (1st), 712 mA h g−1 (2nd) | 100 mA/g, 100 cycles, 440 mA h g−1 | |
| Cauliflower-like WO3 decorated with carbon [110] | Hydrothermal + firing | 67% | 0–3.0 V, 50 mA g−1, 750 mA h g−1 (1st) and 500 mA h g−1 (2nd) | 50 mA/g, 50 cycles, 650 mA h g−1 (~Li5.5WO3) | |
| Carbon-coated 3D WO3 [111] | Template assisted process | 60.1% | 0–3.0 V, C/20, 10,791 mA h g−1 (1st), 649 mA h g−1 (2nd) | ---, 500 cycles, 253 mA h g−1 | |
| WO3*0.33H2O@C nanoparticles [112] | Low temperature combustion | 46.1% | 0–3.0 V, 100 mA g−1, 1543 mA h g−1 (1st) | 100 mA g−1, 200 cycles, 816 mA h g−1 | |
| Ultrathin WO3−x/C nanosheets [113] | Acid-assisted one-pot process | 39.4% | 0–3.0 V, 200 mA g−1, 1866 mA h g−1 (1st), 893 mA h g−1 (2nd) | 200 mA g−1, 100 cycles, 662 mA h g−1 | |
| Products and Structures | Method | Electro-Chromic Energy Storage Type | Electrochromic Performances | Energy Storage Capacity (C) | Cycling Performances | ||
|---|---|---|---|---|---|---|---|
| Optical Transmittance Modulation (▲T) | Switching Time (tc, tb)/s | Color Efficiency/(cm2/C) | |||||
| WO3 nanosheets [167] | Hydrothermal | ECSC | 64.5% (633 nm) | 6.6, 3.8 | 48.9 | 14.9 mF/cm2 | 1000 cycles, ▲T 83.7% retained C 84.5% retained |
| WO3·H2O nanosheet [168] | Hydrothermal | ECSC | 79.0% (633 nm) | 10.1, 6.1 | 42.6 | 43.30 mF/cm2 | 2000 cycles, ▲T 87.8% retained |
| Oxygen-rich nanograin WO3 [169] | Oblique-angle sputtering | ECSC | 82% (630 nm) | ---, --- | ~170 | 0.25 A g−1, 228 F g−1 | 2000 cycles, C 75% retained |
| Mesoporous WO3 film [161] | Sol-gel | ECB | 75.6% (633 nm) | 2.4, 1.2 | 79.7 | 75.3 m A h g−1 | ------ |
| Nb-doped WO3 film [170] | Sol-gel | ECB | 61.7% (633 nm) | 3.6, 2.1 | 49.7 | 74.4 m A h g−1 | 1000 cycles, ▲T 76.2% retained, C 75.8% retained |
| Mo-doped WO3 nanowire arrays [171] | Hydrothermal | ECB | 56.7% (750 nm), 83.0% (1600 nm) | 3.2, 2.6 (750 nm) | 123.5 (750 nm) | 55.89 m A h g−1 | 3500 cycles, ▲T 57.3% retained; 4 A/g, 5500 cycles, C 38.2% retained |
| Amorphous Mo-doped WO3 films [162] | Electrodeposition | ECSC | 83.3% (633 nm) | 2.1, 2.0 | 86.1 | 0.25 mA/cm2, 117.1 mF/cm2 (334.6 m F g−1) | 4000 s, ▲T no obvious change 1500 cycles, C 83% retained |
| PANI/WO3 nanocomposite [172] | Electropolymerization + annealing | ECSC | 35.3% (633 nm) | 13.6, 9.9 | 98.4 | 5 mV/s, 0.025 F/cm2 | 1000 cycles, charge density did not change too much |
| WO3/PANI nanocomposite [173] | Chemical bath | ECSC | Color changes: brownish green-transparent-light green-brownish green | ---, --- | --- | 0.02 mA/cm2, 4.1 mF/cm2 | 800 cycles, C 38% retained |
| Urchin-like WO3@PANI [174] | Solvothermal + electropolymerization | ECB | 45% (700 nm) | 1.9, 1.5 | --- | ---, 831 mA h g−1 | 1200 cycles, 516 mA h/g |
| Honeycombed porous poly(5-formylindole)/WO3 nanocomposites [175] | Hydrothermal + electrochemical polymerization | ECSC | 26% (505 nm); 46% (745 nm) | ---, --- | 137 | ---, 34.1 mF/cm2 | 5000 cycles, C 93% retained |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, W.; Shi, Q.; Hu, R. Advances in Electrochemical Energy Devices Constructed with Tungsten Oxide-Based Nanomaterials. Nanomaterials 2021, 11, 692. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11030692
Han W, Shi Q, Hu R. Advances in Electrochemical Energy Devices Constructed with Tungsten Oxide-Based Nanomaterials. Nanomaterials. 2021; 11(3):692. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11030692
Chicago/Turabian StyleHan, Wenfang, Qian Shi, and Renzong Hu. 2021. "Advances in Electrochemical Energy Devices Constructed with Tungsten Oxide-Based Nanomaterials" Nanomaterials 11, no. 3: 692. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11030692