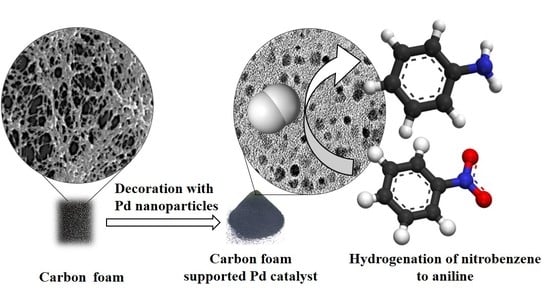
Development of Highly Efficient, Glassy Carbon Foam Supported, Palladium Catalysts for Hydrogenation of Nitrobenzene
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of Glassy Carbon Foam Catalyst Supports
3.2. Characterization of the Palladium-Decorated Glassy Carbon Foam Samples
3.3. Catalytic Tests of the Glassy Carbon Foam Catalysts Containing Palladium
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rakitin, M.Y.; Doluda, V.Y.; Tereshchenkov, A.Y.; Demidenko, G.N.; Lakina, N.V.; Matveeva, V.G.; Sul’man, M.G.; Sul’man, E.M. Investigating the catalytic hydrogenation of nitrobenzene in supercritical carbon dioxide using Pd-containing catalysts. Catal. Ind. 2015, 7, 1–5. [Google Scholar] [CrossRef]
- Qu, R.; Macino, M.; Iqbal, S.; Gao, X.; He, Q.; Hutchings, G.J.; Sankar, M. Supported bimetallic AuPd nanoparticles as a catalyst for the selective hydrogenation of nitroarenes. Nanomaterials 2018, 8, 690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Wen, G.; Zhong, B.; Zhang, B.; Gu, X.; Wang, N.; Su, D. Reduction of nitrobenzene catalyzed by carbon materials. Cuihua Xuebao/Chin. J. Catal. 2014, 35, 914–921. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, L.; Shi, W.; Niu, Y.; Zhang, B.; Su, D. Facile-fabricated iron oxide nanorods as a catalyst for hydrogenation of nitrobenzene. Chin. Chem. Lett. 2019, 30, 183–186. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, Z.; He, S.; Hao, F.; Yang, Y.; Lv, Y.; Zhang, W.; Liu, P.; Luo, H. Nitrogen-doped carbon nanotubes as a highly active metal-free catalyst for nitrobenzene hydrogenation. Appl. Catal. B Environ. 2020, 260, 118105. [Google Scholar] [CrossRef]
- Haber, F. Gradual electrolytic reduction of nitrobenzene with limited cathode potential. Z. Elecktrochem. 1898, 4, 506. [Google Scholar]
- Gelder, E.A.; Jackson, S.D.; Lok, C.M. The hydrogenation of nitrobenzene to aniline: A new mechanism. Chem. Commun. 2005, 522–524. [Google Scholar] [CrossRef]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon N. Y. 2001, 39, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Xie, K.-C.; Lv, Y.-K.; Bao, W.-R. Process effects on activated carbon with large specific surface area from corn cob. Bioresour. Technol. 2006, 97, 110–115. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. R Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Ren, X.; Chen, C.; Nagatsu, M.; Wang, X. Carbon nanotubes as adsorbents in environmental pollution management: A review. Chem. Eng. J. 2011, 170, 395–410. [Google Scholar] [CrossRef]
- Oosthuizen, R.S.; Nyamori, V.O. Carbon nanotubes as supports for palladium and bimetallic catalysts for use in hydrogenation reactions. Platin. Met. Rev. 2011, 55, 154–169. [Google Scholar] [CrossRef]
- Fan, G.Y.; Huang, W.J. Solvent-Free Hydrogenation of Nitrobenzene Catalyzed by Magnetically Recoverable Pt Deposited on Multiwalled Carbon Nanotubes. Synth. React. Inorg. Metal-Org. Nano-Met. Chem. 2015, 45, 1819–1825. [Google Scholar] [CrossRef]
- Qu, Y.; Chen, T.; Wang, G. Hydrogenation of nitrobenzene catalyzed by Pd promoted Ni supported on C 60 derivative. Appl. Surf. Sci. 2019, 465, 888–894. [Google Scholar] [CrossRef]
- Jiang, J.; Zhai, R.; Bao, X. Electrocatalytic properties of Cu-Zr amorphous alloy towards the electrochemical hydrogenation of nitrobenzene. J. Alloys Compd. 2003, 354, 248–258. [Google Scholar] [CrossRef]
- Rodríguez-Ramos, I.; Reinoso, F.R.; Guerrero-Ruiz, A.; de Dios López-González, J. Hydrogenation of CO and CO2 on carbon black-supported Ru catalysts. J. Chem. Technol. Biotechnol. 1986, 36, 67–73. [Google Scholar] [CrossRef]
- Villano, R.; Acocella, M.R.; Guerra, G. Oxidized Carbon Black as Catalyst for the Enamine Formation in Solvent-Free Conditions: A Green Strategy to Build the Benzodiazepine Scaffold. ChemistrySelect 2017, 2, 10559–10564. [Google Scholar] [CrossRef]
- Gancarczyk, A.; Macek, W.; Kołodziej, A. Heat transfer phenomena of glassy carbon foams. Chem. Eng. Res. Des. 2019, 143, 1–3. [Google Scholar] [CrossRef]
- Letellier, M.; Macutkevic, J.; Kuzhir, P.; Banys, J.; Fierro, V.; Celzard, A. Electromagnetic properties of model vitreous carbon foams. Carbon N. Y. 2017, 122, 217–227. [Google Scholar] [CrossRef]
- Xu, L.; Wu, J.F.; Bai, S. Boron-doped glassy carbon fabricated by chemical vapor deposition. Xinxing Tan Cailiao/New Carbon Mater. 2012, 27, 226–232. [Google Scholar] [CrossRef]
- Almajali, M.; Lafdi, K.; Prodhomme, P.H.; Ochoa, O. Mechanical properties of copper-coated carbon foams. Carbon N. Y. 2010, 48, 1604–1608. [Google Scholar] [CrossRef]
- Durkić, T.; Perić, A.; Laušević, M.; Dekanski, A.; Nešković, O.; Veljković, M.; Laušević, Z. Boron and phosphorus doped glassy carbon: I. Surface properties. Carbon 1997, 35, 1567–1572. [Google Scholar] [CrossRef]
- Oishi, S.S.; Botelho, E.C.; Rezende, M.C.; Ferreira, N.G. Structural and surface functionality changes in reticulated vitreous carbon produced from poly(furfuryl alcohol) with sodium hydroxide additions. Appl. Surf. Sci. 2017, 394, 87–97. [Google Scholar] [CrossRef]
- Tadyszak, K.; Litowczenko, J.; Majchrzycki, Ł.; Jeżowski, P.; Załęski, K.; Scheibe, B. Sucrose based cellular glassy carbon for biological applications. Mater. Chem. Phys. 2020, 239, 122033. [Google Scholar] [CrossRef]
- Friedrich, J.M.; Ponce-de-León, C.; Reade, G.W.; Walsh, F.C. Reticulated vitreous carbon as an electrode material. J. Electroanal. Chem. 2004, 561, 203–217. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, M.; Qiu, J.; Guo, Q. Carbon foam: Preparation and application. Carbon 2015, 87, 128–152. [Google Scholar] [CrossRef]
- Narasimman, R.; Vijayan, S.; Prabhakaran, K. Carbon particle induced foaming of molten sucrose for the preparation of carbon foams. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2014, 189, 82–89. [Google Scholar] [CrossRef]
- Udayakumar, M.; El Marabate, B.; Koós, T.; Szemmelveisz, K.; Kristály, F.; Leskó, M.; Filep, Á.; Géber, R.; Schabikowski, M.; Baumli, P.; et al. Synthesis of activated carbon foams with high specific surface area using polyurethane elastomer template for effective removal of methylene blue. Arab. J. Chem. 2021. under revision. [Google Scholar]
- Yao, H.-C.; Emmett, P.H. Kinetics of Liquid Phase Hydrogenation. II. Hydrogenation of Aromatic and Aliphatic Nitrocompounds Over A Colloidal Platinum Catalyst. J. Am. Chem. Soc. 1961, 83, 796–799. [Google Scholar] [CrossRef]
- Plomp, A.J.; Su, D.S.; Jong, K.P.D.; Bitter, J.H. On the nature of oxygen-containing surface groups on carbon nanofibers and their role for platinum depositionsan XPS and titration study. J. Phys. Chem. C 2009, 113, 9865–9869. [Google Scholar] [CrossRef]
- Zhong, R.S.; Qin, Y.H.; Niu, D.F.; Zhang, X.S.; Zhou, X.G.; Sun, S.G.; Yuan, W.K. Effect of carbon nanofiber surface groups on oxygen reduction reaction of supported Pt electrocatalyst. Electrochim. Acta 2013, 89, 157–162. [Google Scholar] [CrossRef]
- Chutia, A.; Hamada, I.; Tokuyama, M. A theoretical insight on the interaction between Pt nanoparticles and hydroxylated graphene nanoflakes. Surf. Sci. 2014, 628, 116–125. [Google Scholar] [CrossRef]
- Wang, C.; Yang, F.; Yang, W.; Ren, L.; Zhang, Y.; Jia, X.; Zhang, L.; Li, Y. PdO nanoparticles enhancing the catalytic activity of Pd/carbon nanotubes for 4-nitrophenol reduction. RSC Adv. 2015, 5, 27526–27532. [Google Scholar] [CrossRef]
- Turáková, M.; Salmi, T.; Eränen, K.; Wärnå, J.; Murzin, D.Y.; Králik, M. Liquid phase hydrogenation of nitrobenzene. Appl. Catal. A Gen. 2015, 499, 66–76. [Google Scholar] [CrossRef]
- Easterday, R.; Sanchez-Felix, O.; Losovyj, Y.; Pink, M.; Stein, B.D.; Morgan, D.G.; Rakitin, M.; Doluda, V.Y.; Sulman, M.G.; Mahmoud, W.E.; et al. Design of ruthenium/iron oxide nanoparticle mixtures for hydrogenation of nitrobenzene. Catal. Sci. Technol. 2015, 5, 1902–1910. [Google Scholar] [CrossRef]
- Peureux, J.; Torres, M.; Mozzanega, H.; Giroir-Fendler, A.; Dalmon, J.A. Nitrobenzene liquid-phase hydrogenation in a membrane reactor. Catal. Today 1995, 25, 409–415. [Google Scholar] [CrossRef]
- Kim, J.H.; Cheon, J.Y.; Shin, T.J.; Park, J.Y.; Joo, S.H. Effect of surface oxygen functionalization of carbon support on the activity and durability of Pt/C catalysts for the oxygen reduction reaction. Carbon 2016, 101, 449–457. [Google Scholar] [CrossRef]
- Kim, J.H.; Yuk, Y.; Joo, H.S.; Cheon, J.Y.; Choi, H.S.; Joo, S.H.; Park, J.Y. Nanoscale adhesion between Pt nanoparticles and carbon support and its influence on the durability of fuel cells. Curr. Appl. Phys. 2015, 15, S108–S114. [Google Scholar] [CrossRef]









| Pd/GCF | Pd/GCF-AC1 | Pd/GCF-AC2 | |
|---|---|---|---|
| Mean with Std. (nm) | 8.0 ± 4.3 | 7.6 ± 4.2 | 4.4 ± 1.6 |
| Min. diameter (nm) | 3.1 | 2.8 | 2.3 |
| Max. diameter (nm) | 30.1 | 21.4 | 10.2 |
| 1st quartile (nm) | 5.3 | 4.5 | 3.3 |
| 3rd quartile (nm) | 9.2 | 9.3 | 5.1 |
| 283 K | 293 K | 303 K | 323 K | |
|---|---|---|---|---|
| Pd/GCF | 2.5 × 10−4 ± 1.3 × 10−5 | 5.2 × 10−4 ± 1.6 × 10−5 | 5.7 × 10−4 ± 3.6 × 10−5 | 8.2 × 10−4 ± 6.3 × 10−5 |
| Pd/GCF-AC1 | 5.6 × 10−4 ± 3.4 × 10−5 | 7.3 × 10−4 ± 5.9 × 10−5 | 1.2 × 10−3 ± 1.0 × 10−4 | 1.8 × 10−3 ± 1.3 × 10−4 |
| Pd/GCF-AC2 | 9.7 × 10−4 ± 5.4 × 10−5 | 9.1 × 10−4 ± 7.2 × 10−5 | 1.9 × 10−3 ± 2.0 × 10−4 | 3.2 × 10−3 ± 1.6 × 10−4 |
| Pd/GCF | Pd/GCF-AC1 | Pd/GCF-AC2 | |
|---|---|---|---|
| Ea (kJ ∙ mol−1) | 20.18 ± 4.9 | 21.43 ± 2.9 | 22.9 ± 0.9 |
| Cat. Type | Wt. (g) | c (mol/dm3) | p (bar) | T (K) | t (min) | Yaniline (%) | Ea (kJ/mol) |
|---|---|---|---|---|---|---|---|
| Pd/GCF | 0.10 | 0.25 | 20 | 293 | 180 | 99.2 | 20.18 ± 4.9 |
| Pd/GCF-AC1 | 293 | 120 | 99.0 | 21.43 ± 2.9 | |||
| Pd/GCF-AC2 | 323 | 80 | 98.0 | 22.90 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prekob, Á.; Udayakumar, M.; Karacs, G.; Kristály, F.; Muránszky, G.; Leskó, A.K.; Németh, Z.; Viskolcz, B.; Vanyorek, L. Development of Highly Efficient, Glassy Carbon Foam Supported, Palladium Catalysts for Hydrogenation of Nitrobenzene. Nanomaterials 2021, 11, 1172. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051172
Prekob Á, Udayakumar M, Karacs G, Kristály F, Muránszky G, Leskó AK, Németh Z, Viskolcz B, Vanyorek L. Development of Highly Efficient, Glassy Carbon Foam Supported, Palladium Catalysts for Hydrogenation of Nitrobenzene. Nanomaterials. 2021; 11(5):1172. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051172
Chicago/Turabian StylePrekob, Ádám, Mahitha Udayakumar, Gábor Karacs, Ferenc Kristály, Gábor Muránszky, Anett Katalin Leskó, Zoltán Németh, Béla Viskolcz, and László Vanyorek. 2021. "Development of Highly Efficient, Glassy Carbon Foam Supported, Palladium Catalysts for Hydrogenation of Nitrobenzene" Nanomaterials 11, no. 5: 1172. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051172