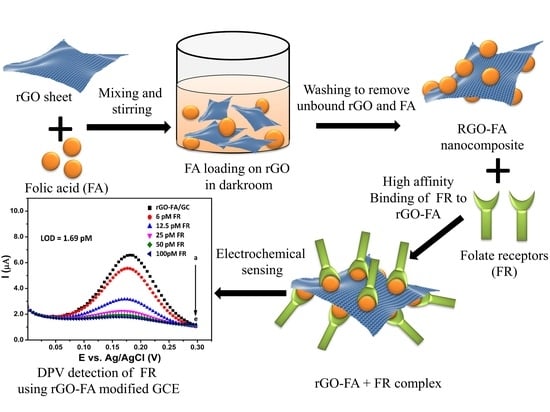
Highly Sensitive Electrochemical Biosensor Using Folic Acid-Modified Reduced Graphene Oxide for the Detection of Cancer Biomarker
Abstract
:1. Introduction
2. Scope of the Study
3. Experimental Section
3.1. Materials
3.2. Synthesis of rGO
3.3. Synthesis of rGO-FA
3.4. Characterization Techniques
3.5. Electrochemical Measurements
4. Results and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, H.-Y.; Liu, S.-M.; Zhang, Y.-Z. Maternal Folic Acid Supplementation Mediates Offspring Health via DNA Methylation. Reprod. Sci. 2020, 27, 963–976. [Google Scholar] [CrossRef]
- Ye, S.; Zhou, X.; Chen, P.; Lin, J.-F. Folic acid attenuates remodeling and dysfunction in the aging heart through the ER stress pathway. Life Sci. 2021, 264, 118718. [Google Scholar] [CrossRef]
- Lucock, M. Folic Acid: Nutritional Biochemistry, Molecular Biology, and Role in Disease Processes. Mol. Genet. Metab. 2000, 71, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J. Nutr. Heal. Aging 2002, 6, 39–42. [Google Scholar]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, folate, and the methionine remethylation cycle—biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinlivan, E.P.; Gregory, J.F. Effect of food fortification on folic acid intake in the United States. Am. J. Clin. Nutr. 2003, 77, 221–225. [Google Scholar] [CrossRef]
- Ebbing, M.; Bønaa, K.H.; Nygård, O.; Arnesen, E.; Ueland, P.M.; Nordrehaug, J.E.; Rasmussen, K.; Njølstad, I.; Refsum, H.; Nilsen, D.W. Cancer incidence and mortality after treatment with folic acid and vitamin B12. J. Am. Med Assoc. (JAMA) 2009, 302, 2119–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Broin, J.D.; Temperley, I.J.; Brown, J.P.; Scott, J.M. Nutritional stability of various naturally occurring monoglutamate derivatives of folic acid. Am. J. Clin. Nutr. 1975, 28, 438–444. [Google Scholar] [CrossRef]
- Arzeni, C.; Pérez, O.E.; LeBlanc, J.G.; Pilosof, A.M. Egg albumin–folic acid nanocomplexes: Performance as a functional ingredient and biological activity. J. Funct. Foods 2015, 18, 379–386. [Google Scholar] [CrossRef]
- Ledermann, J.; Canevari, S.; Thigpen, T. Targeting the folate receptor: Diagnostic and therapeutic approaches to personalize cancer treatments. Ann. Oncol. 2015, 26, 2034–2043. [Google Scholar] [CrossRef]
- Zhou, Y.; Unno, K.; Hyjek, E.; Liu, H.; Zimmerman, T.; Karmakar, S.; Putt, K.S.; Shen, J.; Low, P.S.; Wickrema, A. Expression of functional folate receptors in multiple myeloma. Leuk. Lymphoma 2018, 59, 2982–2989. [Google Scholar] [CrossRef]
- Luangwattananun, P.; Junking, M.; Sujjitjoon, J.; Wutti-In, Y.; Poungvarin, N.; Thuwajit, C.; Yenchitsomanus, P.-T. Fourth-generation chimeric antigen receptor T cells targeting folate receptor alpha antigen expressed on breast cancer cells for adoptive T cell therapy. Breast Cancer Res. Treat. 2021, 186, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.; Bruun, S.W.; Hansen, S.I. The complex interplay between ligand binding and conformational structure of the folate binding protein (folate receptor): Biological perspectives. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2015, 1854, 1249–1259. [Google Scholar] [CrossRef]
- Vergote, I.B.; Marth, C.; Coleman, R.L. Role of the folate receptor in ovarian cancer treatment: Evidence, mechanism, and clinical implications. Cancer Metastasis Rev. 2015, 34, 41–52. [Google Scholar] [CrossRef]
- O’Shannessy, D.J.; Somers, E.B.; Wang, L.-C.; Wang, H.; Hsu, R. Expression of folate receptors alpha and beta in normal and cancerous gynecologic tissues: Correlation of expression of the beta isoform with macrophage markers. J. Ovarian Res. 2015, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cresswell, G.M. Folate Receptor Beta as a Marker of Immunosuppressive Myeloid Derived Suppressor Cells and Tumor Associated Macrophages in the Tumor Microenvironment. Ph.D. Thesis, Purdue University, West Lafayette, IN, USA, 2018. [Google Scholar]
- Chandrupatla, D.M.S.H.; Molthoff, C.F.M.; Lammertsma, A.A.; Van Der Laken, C.J.; Jansen, G. The folate receptor β as a macrophage-mediated imaging and therapeutic target in rheumatoid arthritis. Drug Deliv. Transl. Res. 2019, 9, 366–378. [Google Scholar] [CrossRef] [Green Version]
- Cresswell, G.M.; Wang, B.; Kischuk, E.M.; Broman, M.M.; Alfar, R.A.; Vickman, R.E.; Dimitrov, D.S.; Kularatne, S.A.; Sundaram, C.P.; Singhal, S.; et al. Folate Receptor Beta Designates Immunosuppressive Tumor-Associated Myeloid Cells That Can Be Reprogrammed with Folate-Targeted Drugs. Cancer Res. 2021, 81, 671–684. [Google Scholar] [CrossRef]
- Nagai, T.; Furusho, Y.; Li, H.; Hasui, K.; Matsukita, S.; Sueyoshi, K.; Yanagi, M.; Hatae, M.; Takao, S.; Matsuyama, T. Production of a High-affinity Monoclonal Antibody Reactive with Folate Receptors Alpha and Beta. Monoclon. Antibodies Immunodiagn. Immunother. 2015, 34, 181–190. [Google Scholar] [CrossRef]
- Xie, J.; Chen, Z.; Zhang, A.; Zheng, B.; Yang, S.; Teng, L. Folate receptor targeted drug delivery- from the bench to the bedside. Eur. J. Biomed. Res. 2016, 2, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Jiang, Y.; Zhang, M.; Fei, X.; Gu, Y. Novel fluorescent antifolates that target folate receptors α and β: Molecular dynamics and density functional theory study. J. Mol. Graph. Model. 2018, 85, 40–47. [Google Scholar] [CrossRef]
- Choi, J.-S.; Park, J.-W.; Seu, Y.-B.; Doh, K.-O. Enhanced efficacy of folate-incorporated cholesteryl doxorubicin liposome in folate receptor abundant cancer cell. J. Drug Deliv. Sci. Technol. 2021, 62, 102385. [Google Scholar] [CrossRef]
- Park, I.Y.; Kwon, S.-H.; Lee, G.; Motoyama, K.; Kim, M.W.; Lin, M.; Niidome, T.; Choi, J.H.; Lee, R. pH-sensitive multi-drug liposomes targeting folate receptor β for efficient treatment of non-small cell lung cancer. J. Control. Release 2021, 330, 1–14. [Google Scholar] [CrossRef]
- Zhao, R.; Min, S.H.; Wang, Y.; Campanella, E.; Low, P.S.; Goldman, I.D. A Role for the Proton-coupled Folate Transporter (PCFT-SLC46A1) in Folate Receptor-mediated Endocytosis. J. Biol. Chem. 2009, 284, 4267–4274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Ke, J.; Zhou, X.E.; Yi, W.; Brunzelle, J.S.; Li, J.; Yong, E.-L.; Xu, H.E.; Melcher, K. Structural basis for molecular recognition of folic acid by folate receptors. Nat. Cell Biol. 2013, 500, 486–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basal, E.; Eghbali-Fatourechi, G.Z.; Kalli, K.R.; Hartmann, L.C.; Goodman, K.M.; Goode, E.L.; Kamen, B.A.; Low, P.S.; Knutson, K.L. Functional Folate Receptor Alpha Is Elevated in the Blood of Ovarian Cancer Patients. PLoS ONE 2009, 4, e6292. [Google Scholar] [CrossRef] [PubMed]
- van Zijl, F.; Krupitza, G.; Mikulits, W. Initial steps of metastasis: Cell invasion and endothelial transmigration. Mutat. Res. Mutat. Res. 2011, 728, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Ramzy, L.; Nasr, M.; Metwally, A.A.; Awad, G.A. Cancer nanotheranostics: A review of the role of conjugated ligands for overexpressed receptors. Eur. J. Pharm. Sci. 2017, 104, 273–292. [Google Scholar] [CrossRef]
- Fernández, M.; Javaid, F.; Chudasama, V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Huang, P.; He, M.; Pan, L.; Zhou, Z.; Feng, L.; Gao, G.; Cui, D. Folic acid-conjugated LaF3: Yb, Tm@ SiO2 nanoprobes for targeting dual-modality imaging of upconversion luminescence and X-ray computed tomography. J. Phys. Chem. B 2012, 116, 14062–14070. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, L.; Peng, C.; Shen, M.; Shi, X.; Zhang, G. Folic acid-modified dendrimer-entrapped gold nanoparticles as nanoprobes for targeted CT imaging of human lung adenocarcinoma. Biomaterials 2013, 34, 470–480. [Google Scholar] [CrossRef]
- Wang, M.; Hu, H.; Sun, Y.; Qiu, L.; Zhang, J.; Guan, G.; Zhao, X.; Qiao, M.; Cheng, L.; Cheng, L. A pH-sensitive gene delivery system based on folic acid-PEG-chitosan–PAMAM-plasmid DNA complexes for cancer cell targeting. Biomaterials 2013, 34, 10120–10132. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Shim, Y.-H.; Oh, J.-S.; Jeong, Y.-I.; Park, I.-K.; Lee, H.C. Folic-acid-conjugated pullulan/poly(DL-lactide-co-glycolide) graft copolymer nanoparticles for folate-receptor-mediated drug delivery. Nanoscale Res. Lett. 2015, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghalehkhondabi, V.; Soleymani, M.; Fazlali, A. Folate-targeted nanomicelles containing silibinin as an active drug delivery system for liver cancer therapy. J. Drug Deliv. Sci. Technol. 2021, 61, 102157. [Google Scholar] [CrossRef]
- Stella, B.; Arpicco, S.; Peracchia, M.T.; Desmaële, D.; Hoebeke, J.; Renoir, M.; D’Angelo, J.; Cattel, L.; Couvreur, P. Design of Folic Acid-Conjugated Nanoparticles for Drug Targeting. J. Pharm. Sci. 2000, 89, 1452–1464. [Google Scholar] [CrossRef]
- Qin, X.; Guo, Z.; Liu, Z.; Zhang, W.; Wan, M.; Yang, B. Folic acid-conjugated graphene oxide for cancer targeted chemo-photothermal therapy. J. Photochem. Photobiol. B Biol. 2013, 120, 156–162. [Google Scholar] [CrossRef]
- Chan, M.-H.; Lin, H.-M. Preparation and identification of multifunctional mesoporous silica nanoparticles for in vitro and in vivo dual-mode imaging, theranostics, and targeted tracking. Biomaterials 2015, 46, 149–158. [Google Scholar] [CrossRef]
- Alibolandi, M.; Abnous, K.; Sadeghi, F.; Hosseinkhani, H.; Ramezani, M.; Hadizadeh, F. Folate receptor-targeted multimodal polymersomes for delivery of quantum dots and doxorubicin to breast adenocarcinoma: In vitro and in vivo evaluation. Int. J. Pharm. 2016, 500, 162–178. [Google Scholar] [CrossRef]
- Karpuz, M.; Silindir-Gunay, M.; Ozer, A.Y.; Ozturk, S.C.; Yanik, H.; Tuncel, M.; Aydin, C.; Esendagli, G. Diagnostic and therapeutic evaluation of folate-targeted paclitaxel and vinorelbine encapsulating theranostic liposomes for non-small cell lung cancer. Eur. J. Pharm. Sci. 2021, 156, 105576. [Google Scholar] [CrossRef]
- Soleymani, J.; Hasanzadeh, M.; Somi, M.H.; Ozkan, S.A.; Jouyban, A. Targeting and sensing of some cancer cells using folate bioreceptor functionalized nitrogen-doped graphene quantum dots. Int. J. Biol. Macromol. 2018, 118, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Knežević, N.Ž.; Gadjanski, I.; Durand, J.-O. Magnetic nanoarchitectures for cancer sensing, imaging and therapy. J. Mater. Chem. B 2018, 7, 9–23. [Google Scholar] [CrossRef]
- Roy, S.; Gunukula, A.; Ghosh, B.; Chakraborty, C. A folic acid-sensitive polyfluorene based “turn-off” fluorescence nanoprobe for folate receptor overexpressed cancer cell imaging. Sens. Actuators B Chem. 2019, 291, 337–344. [Google Scholar] [CrossRef]
- Teixeira, R.A.R.; Lima, F.R.A.; Silva, P.C.; Costa, L.A.S.; Sant’Ana, A.C. Tracking chemical interactions of folic acid on gold surface by SERS spectroscopy. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2019, 223, 117305. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, J.; Hasanzadeh, M.; Shadjou, N.; Somi, M.H.; Jouyban, A. The role of nanomaterials on the cancer cells sensing based on folate receptor: Analytical approach. TrAC Trends Anal. Chem. 2020, 125, 115834. [Google Scholar] [CrossRef]
- Castillo, J.J.; Svendsen, W.E.; Rozlosnik, N.; Escobar, P.; Martínez, F.; León, J.C. Detection of cancer cells using a peptide nanotube–folic acid modified graphene electrode. Analyst 2013, 138, 1026–1031. [Google Scholar] [CrossRef]
- Reddy, J.A.; Low, P.S. Folate-mediated targeting of therapeutic and imaging agents to cancers. Crit. Rev. Ther. Drug Carr. Syst. 1998, 15, 41. [Google Scholar] [CrossRef]
- Bai, G.R.; Muthoosamy, K.; Shipton, F.N.; Pandikumar, A.; Rameshkumar, P.; Huang, N.M.; Manickam, S. The bio-genic synthesis of a reduced graphene oxide–silver (RGO–Ag) nanocomposite and its dual applications as an antibacterial agent and cancer biomarker sensor. RSC Adv. 2016, 6, 36576–36587. [Google Scholar]
- Bai, R.G.; Muthoosamy, K.; Zhou, M.; Ashokkumar, M.; Huang, N.M.; Manickam, S. Sonochemical and sustainable synthesis of graphene-gold (G-Au) nanocomposites for enzymeless and selective electrochemical detection of nitric oxide. Biosens. Bioelectron. 2017, 87, 622–629. [Google Scholar] [CrossRef]
- Bai, R.G.; Ninan, N.; Muthoosamy, K.; Manickam, S. Graphene: A versatile platform for nanotheranostics and tissue engineering. Prog. Mater. Sci. 2018, 91, 24–69. [Google Scholar] [CrossRef]
- Bai, R.G.; Muthoosamy, K.; Manickam, S.; Hilal-Alnaqbi, A. Graphene-based 3D scaffolds in tissue engineering: Fabrication, applications, and future scope in liver tissue engineering. Int. J. Nanomed. 2019, 14, 5753–5783. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene Based Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Mao, H.Y.; Laurent, S.; Chen, W.; Akhavan, O.; Imani, M.; Ashkarran, A.A.; Mahmoudi, M. Graphene: Promises, Facts, Opportunities, and Challenges in Nanomedicine. Chem. Rev. 2013, 113, 3407–3424. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, W.J. Photothermally controlled gene delivery by reduced graphene oxide–polyethylenimine nanocomposite. Small 2014, 10, 117–126. [Google Scholar] [CrossRef]
- Muthoosamy, R.G.B.A.S.M.K.; Bai, R.; Manickam, S. Graphene and graphene oxide as a docking station for modern drug delivery system. Curr. Drug Deliv. 2014, 11, 701–718. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Liu, Z. The advancing uses of nano-graphene in drug delivery. Expert Opin. Drug Deliv. 2014, 12, 601–612. [Google Scholar] [CrossRef]
- Benvidi, A.; Saucedo, N.M.; Ramnani, P.; Villarreal, C.; Mulchandani, A.; Tezerjani, M.D.; Jahanbani, S. Electro-oxidised monolayer CVD graphene film transducer for ultrasensitive impedimetric DNA biosensor. Electroanalysis 2018, 30, 1791–1800. [Google Scholar] [CrossRef]
- Ismail, N.A.B.; Abd-Wahab, F.; Salim, W.W.A.W. Cyclic voltammetry and electrochemical impedance spectroscopy of partially reduced graphene oxide-PEDOT: PSS transducer for biochemical sensing. In Proceedings of the 2018 IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES), Sarawak, Malaysia, 3–6 December 2018. [Google Scholar]
- Morales-Narváez, E.; Merkoçi, A. Graphene Oxide as an Optical Biosensing Platform. Adv. Mater. 2012, 24, 3298–3308. [Google Scholar] [CrossRef] [PubMed]
- Muthoosamy, K.; Bai, R.G.; Abubakar, I.B.; Sudheer, S.M.; Lim, H.N.; Loh, H.S.; Huang, N.M.; Chia, C.H.; Manickam, S. Exceedingly biocompatible and thin-layered reduced graphene oxide nanosheets using an eco-friendly mushroom extract strategy. Int. J. Nanomed. 2015, 10, 1505. [Google Scholar]
- Bai, R.G.; Muthoosamy, K.; Shipton, F.N.; Manickam, S. Acoustic cavitation induced generation of stabilizer-free, extremely stable reduced graphene oxide nanodispersion for efficient delivery of paclitaxel in cancer cells. Ultrason. Sonochem. 2017, 36, 129–138. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Huang, X.; Ma, Y.; Huang, Y.; Yang, R.; Duan, H.; Chen, Y. Multi-functionalised graphene oxide based anticancer drug-carrier with dual-targeting function and pH-sensitivity. J. Mater. Chem. 2011, 21, 3448–3454. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Fu, Y.-Y.; Peng, Q.; Guo, S.-S.; Liu, G.; Li, J.; Yang, H.-H.; Chen, G.-N. Dye-enhanced graphene oxide for photothermal therapy and photoacoustic imaging. J. Mater. Chem. B 2013, 1, 5762–5767. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; Lao, J.; He, H.; Cheng, T.; Wang, M.; Wang, S.; Huang, F. Multifunctional graphene quantum dots for simultaneous targeted cellular imaging and drug delivery. Colloids Surf. B Biointerfaces 2014, 122, 638–644. [Google Scholar] [CrossRef]
- Depan, D.; Shah, J.; Misra, R. Controlled release of drug from folate-decorated and graphene mediated drug delivery system: Synthesis, loading efficiency, and drug release response. Mater. Sci. Eng. C 2011, 31, 1305–1312. [Google Scholar] [CrossRef]
- Chen, W.; Cai, S.; Ren, Q.Q.; Wen, W.; Zhao, Y.D. Recent advances in electrochemical sensing for hydrogen peroxide: A review. Analyst 2012, 137, 49–58. [Google Scholar] [CrossRef]
- Marín, S.; Merkoçi, A. Nanomaterials Based Electrochemical Sensing Applications for Safety and Security. Electroanalysis 2012, 24, 459–469. [Google Scholar] [CrossRef]
- Doucette, M.M.; Stevens, V.L. Folate receptor function is regulated in response to different cellular growth rates in cultured mammalian cells. J. Nutr. 2001, 131, 2819–2825. [Google Scholar] [CrossRef] [PubMed]
- Ming, N.H.; Lim, H.; Chia, C.; Yarmo, M.; Muhamad, M. Simple room-temperature preparation of high-yield large-area graphene oxide. Int. J. Nanomed. 2010, 6, 3443–3448. [Google Scholar]
- Matias, R.; Ribeiro, P.R.S.; Sarraguça, M.; Lopes, J.A. A UV spectrophotometric method for the determination of folic acid in pharmaceutical tablets and dissolution tests. Anal. Methods 2014, 6, 3065–3071. [Google Scholar] [CrossRef]
- Sivakumar, C.; Gopalan, A.; Vasudevan, T.; Wen, T.C. Kinetics of polymerization of N-methyl aniline using UV-Vis spectroscopy. Synth. Met. 2002, 126, 123–135. [Google Scholar] [CrossRef]
- Baset, S.; Akbari, H.; Zeynali, H.; Shafie, M. Size measurement of metal and semiconductor nanoparticles via UV-Vis absorption spectra. Digest J. Nanomater. Biostruct. (DJNB) 2011, 6, 709–716. [Google Scholar]
- Mohammed, E.M.K.A.D. Qualitative and quantitative determination of folic acid in tablets by FTIR spectroscopy. Int. J. Adv. Pharm. Biol. Chem. 2014, 3, 773–780. [Google Scholar]
- Vora, A.; Riga, A.; Dollimore, D.; Alexander, K. Thermal Stability of Folic Acid in the solid-state. J. Therm. Anal. Calorim. 2004, 75, 709–717. [Google Scholar] [CrossRef]
- Vora, A.; Riga, A.; Dollimore, D.; Alexander, K.S. Thermal stability of folic acid. Thermochim. Acta 2002, 392, 209–220. [Google Scholar] [CrossRef]
- Hueso, J.; Espinós, J.; Caballero, A.; Cotrino, J.; González-Elipe, A. XPS investigation of the reaction of carbon with NO, O2, N2 and H2O plasmas. Carbon 2007, 45, 89–96. [Google Scholar] [CrossRef]
- Yang, G.; Cao, J.; Li, L.; Rana, R.K.; Zhu, J.J. Carboxymethyl chitosan-functionalized graphene for label-free electrochemical cytosensing. Carbon 2013, 51, 124–133. [Google Scholar] [CrossRef]
- Hu, Y.; Li, F.; Bai, X.; Li, D.; Hua, S.; Wang, K.; Niu, L. Label-free electrochemical impedance sensing of DNA hybridisation based on functionalized graphene sheets. Chem. Commun. 2011, 47, 1743–1745. [Google Scholar] [CrossRef]
- Hao, C.; Ding, L.; Zhang, X.; Ju, H. Biocompatible conductive architecture of carbon nanofiber-doped chitosan prepared with controllable electrodeposition for cytosensing. Anal. Chem. 2007, 79, 4442–4447. [Google Scholar] [CrossRef]
- Wu, Z.; Zhen, Z.; Jiang, J.-H.; Shen, G.-L.; Yu, R.-Q. Terminal protection of small-molecule-linked DNA for sensitive electrochemical detection of protein binding via selective carbon nanotube assembly. J. Am. Chem. Soc. 2009, 131, 12325–12332. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; He, X.; Wang, L.; Zhang, X. A folate receptor electrochemical sensor based on terminal protection and supersandwich DNAzyme amplification. Biosens. Bioelectron. 2013, 42, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.-T.; Zhang, R.; Zou, L.; Zhu, J.-J. A label-free cytosensor for the enhanced electrochemical detection of cancer cells using polydopamine-coated carbon nanotubes. Analyst 2011, 137, 1316–1318. [Google Scholar] [CrossRef]
- Wei, X.; Lin, W.; Ma, N.; Luo, F.; Lin, Z.; Guo, L.; Qiu, B.; Chen, G. Sensitive fluorescence biosensor for folate receptor based on terminal protection of small-molecule-linked DNA. Chem. Commun. 2012, 48, 6184. [Google Scholar] [CrossRef]
- Wang, R.; Di, J.; Ma, J.; Ma, Z. Highly sensitive detection of cancer cells by electrochemical impedance spectroscopy. Electrochim. Acta 2012, 61, 179–184. [Google Scholar] [CrossRef]
- Weng, J.; Zhang, Z.; Sun, L.; Wang, J.A. High sensitive detection of cancer cell with a folic acid-based boron-doped diamond electrode using an AC impedimetric approach. Biosens. Bioelectron. 2011, 26, 1847–1852. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, X.; Zhang, D.; Huang, J.; Li, G. An electrochemical method to detect folate receptor positive tumor cells. Electrochem. Commun. 2007, 9, 2547–2550. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, J.; Lai, Y.; Ma, Y.; Weng, J.; Sun, L. Conjugating folic acid to gold nanoparticles through glutathione for targeting and detecting cancer cells. Bioorg. Med. Chem. 2010, 18, 5528–5534. [Google Scholar] [CrossRef]
- Xu, S.; Liu, J.; Wang, T.; Li, H.; Miao, Y.; Liu, Y.; Wang, J.; Wang, E. A simple and rapid electrochemical strategy for non-invasive, sensitive and specific detection of cancerous cell. Talanta 2013, 104, 122–127. [Google Scholar] [CrossRef]
- Huang, X.; Peng, X.; Wang, Y.; Wang, Y.; Shin, D.M.; El Sayed, M.A.; Nie, S. A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano 2010, 4, 5887–5896. [Google Scholar] [CrossRef] [Green Version]
- O’shannessy, D.J.; Somers, E.B.; Albone, E.; Cheng, X.; Park, Y.C.; Tomkowicz, B.E.; Hamuro, Y.; Kohl, T.O.; Forsyth, T.M.; Smale, R. Characterisation of the human folate receptor alpha via novel antibody-based probes. Oncotarget 2011, 2, 1227–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.B.; Zhang, H.D.; Xu, S.P.; Gan, T.; Huang, K.J.; Liu, Y.M. A sensitive and label-free electrochemical impedance biosensor for protein detection based on terminal protection of small molecule-linked DNA. Sens. Actuators B Chem. 2014, 194, 478–483. [Google Scholar] [CrossRef]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef] [PubMed]









| Material | Carbon (wt%) | Oxygen (wt%) | Nitrogen (wt%) |
|---|---|---|---|
| rGO | 59.41 | 40.59 | - |
| FA | 62.26 | 28.21 | 9.53 |
| rGO-FA | 68.70 | 18.05 | 13.25 |
| Sensing Material | Working Electrode | Material or Cell Line Used and Range of Detection | Limit of Detection (FR Conc. or Number of Cells/mL) | Method | Reference |
|---|---|---|---|---|---|
| FA-DNA–SWNT | Au | FR (0.01–10 nM) | 3 pM | DPV | [79] |
| FA-DNA | Au | FR (1.0–20.0 ng/mL) | 0.3 ng/mL | CV SSA | [80] |
| CNTs@PDA-FA | GC | HL-60 cells (5 × 103–5 × 105 cells/mL) | 5 × 102 cells | EIS | [81] |
| MPA/(Fc-PEI/SWNT) | Au | HeLa cells (10–106 cells/mL) | 10 cells | DPV | [62] |
| PNT–FA | G | HeLa cells (250–5 × 103 cells/ mL) | 250 cells | CV | [82] |
| PNT–FA | G | FR (8–13 nM) | 8 nM | CV | [82] |
| Au/MUA-FA | Au | HeLa cells (6–105 cells/mL) | 6 cells | EIS | [83] |
| Au-FA | BDD | HeLa cells (10–105 cells/ mL) | 10 cells | EIS | [84] |
| FA-AuNPs | Au | Hela cells (1.3 × 105) | Not indicated | CV | [85] |
| FA-GSH-GNPs | - | HeLa cells (10–105 cells/mL) | 100 cells | Absorbance | [86] |
| FA/PEI/CMC-G | GC | HL-60 cells (500–5 × 106 cells/ mL) | 500 cells | EIS | [76] |
| FA- MHDA-HT-Fc | Au beads | HeLa cells (10–106 cells/mL) | 10 cells | DPV | [87] |
| rGO-FA | GC | FR (6–100 pM) | 1.69 pM | DPV | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geetha Bai, R.; Muthoosamy, K.; Tuvikene, R.; Nay Ming, H.; Manickam, S. Highly Sensitive Electrochemical Biosensor Using Folic Acid-Modified Reduced Graphene Oxide for the Detection of Cancer Biomarker. Nanomaterials 2021, 11, 1272. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051272
Geetha Bai R, Muthoosamy K, Tuvikene R, Nay Ming H, Manickam S. Highly Sensitive Electrochemical Biosensor Using Folic Acid-Modified Reduced Graphene Oxide for the Detection of Cancer Biomarker. Nanomaterials. 2021; 11(5):1272. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051272
Chicago/Turabian StyleGeetha Bai, Renu, Kasturi Muthoosamy, Rando Tuvikene, Huang Nay Ming, and Sivakumar Manickam. 2021. "Highly Sensitive Electrochemical Biosensor Using Folic Acid-Modified Reduced Graphene Oxide for the Detection of Cancer Biomarker" Nanomaterials 11, no. 5: 1272. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051272