Fabrication, Microstructure and Colloidal Stability of Humic Acids Loaded Fe3O4/APTES Nanosorbents for Environmental Applications
Abstract
:1. Introduction
2. Materials and Methods
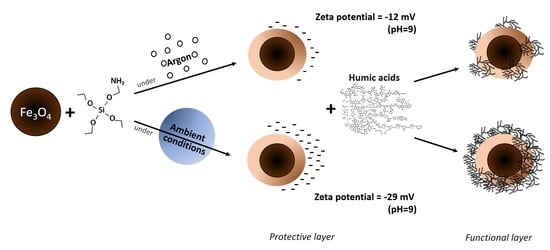
2.1. Synthesis of Fe3O4 MNPs
2.2. Synthesis of Fe3O4/APTES MNPs
2.3. Humic Acids Characterization
2.4. Characterization of Samples
3. Results
3.1. Microstructure of Fe3O4/APTES MNPs
3.2. Characteristics of Surface Charging and Hydrodynamic Size of MNPs
3.2.1. Effects of APTES and Oxidation
3.2.2. Effects of HA Adsorption on MNPs Charge
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mohammed, L.; Gomaa, H.G.; Rajab, D.; Zhu, J. Magnetic Nanoparticles for Environmental and Biomedical Applications: A Review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Jiang, B.; Lian, L.; Xing, Y.; Zhang, N.; Chen, Y.; Lu, P.; Zhang, D. Advances of Magnetic Nanoparticles in Environmental Application: Environmental Remediation and (Bio)sensors as Case Studies. Environ. Sci. Pollut. Res. 2018, 25, 30863–30879. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Lo, I. Magnetic Nanoparticles: Essential Factors for Sustainable Environmental Applications. Water Res. 2013, 47, 2613–2632. [Google Scholar] [CrossRef] [PubMed]
- Majewski, P.; Thierry, B. Functionalized Magnetite Nanoparticles—Synthesis, Properties, and Bio-Applications. Crit. Rev. Solid State Mater. Sci. 2007, 32, 203–215. [Google Scholar] [CrossRef]
- Tombácz, E.; Turcu, R.; Socoliuc, V.; Vékás, L. Magnetic Iron Oxide Nanoparticles: Recent Trends in Design and Synthesis of Magnetoresponsive Nanosystems. Biochem. Biophys. Res. Commun. 2015, 468, 442–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallyn, J.; Anton, N.; Vandamme, T. Synthesis, Principles, and Properties of Magnetite Nanoparticles for in vivo Imaging Applications—A Review. Pharmaceutics 2019, 11, 601. [Google Scholar] [CrossRef] [Green Version]
- Barrera, G.; Tiberto, P.; Allia, P.; Bonelli, B.; Esposito, S.; Marocco, A.; Michele, P.; Leterrier, Y. Magnetic Properties of Nanocomposites. Appl. Sci. 2019, 9, 212. [Google Scholar] [CrossRef] [Green Version]
- Esposito, S.; Marocco, A.; Dell’Agli, G.; Bonelli, B.; Mannu, F.; Allia, P.; Tiberto, P.; Barrera, G.; Pansini, M. Separation of Biological Entities from Human Blood by Using Magnetic Nanocomposites Obtained from Zeolite Precursors. Molecules 2020, 25, 1803. [Google Scholar] [CrossRef] [Green Version]
- Peddis, D.; Laureti, S.; Fiorani, D. New Trends in Nanoparticle Magnetism; Springer Series in Materials Science; Springer: Cham, Switzerland, 2021; 440p. [Google Scholar] [CrossRef]
- Illés, E.; Tombácz, E. The Effect of Humic Acid Adsorption on pH-Dependent Surface Charging and Aggregation of Magnetite Nanoparticles. J. Colloid Interface Sci. 2006, 295, 115–123. [Google Scholar] [CrossRef]
- Mallakpour, S.; Madani, M.A. Review of Current Coupling Agents for Modification of Metal Oxide Nanoparticles. Prog. Org. Coat. 2015, 86, 194–207. [Google Scholar] [CrossRef]
- Kydralieva, K.A.; Yurishcheva, A.A.; Dzhardimalieva, G.I.; Jorobekova, S.J. Nanoparticles of Magnetite in Polymer Matrices: Synthesis and Properties. J. Inorg. Organomet. Polym. Mater. 2016, 26, 1212–1223. [Google Scholar] [CrossRef]
- Liu, I.F.; Zhao, Z.S.; Jiang, G.B. Coating Fe3O4 Magnetic Nanoparticles with Humic Acid for High Efficient Removal of Heavy Metals in Water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef] [PubMed]
- Sundman, A.; Vitzhum, A.-L.; Adaktylos-Surber, K.; Figueroa, A.I.; van der Laan, G.; Daus, B.; Byrne, J.M. Effect of Fe-metabolizing Bacteria and Humic Substances on Magnetite Nanoparticle Reactivity towards Arsenic and Chromium. J. Hazard. Mater. 2019, 384, 121450. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, L.S.; Kovel, E.S.; Kydralieva, K.A.; Dzhardimalieva, G.I.; Illés, E.; Tombácz, E.; Kudryasheva, N.S. Effects of Modified Magnetite Nanoparticles on Bacterial Cells and Enzyme Reactions. Nanomaterials 2020, 10, 1499. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Irzhak, V.I.; Bratskaya, S.Y.; Mayorov, V.Y.; Privar, Y.O.; Kasymova, E.D.; Kulyabko, L.S.; Zhorobekova, S.Z.; Kydralieva, K.A. Stabilization of Magnetite Nanoparticles in Humic Acids Medium and Study of their Sorption Properties. Colloid J. 2020, 82, 1–7. [Google Scholar] [CrossRef]
- Janos, P.; Kormunda, M.; Zivotsky, O.; Pilarova, V. Composite Fe3O4/humic Acid Magnetic Sorbent and its Sorption Ability for Chlorophenols and Some Other Aromatics Compounds. Sep. Sci. Technol. 2013, 48, 2028–2035. [Google Scholar] [CrossRef]
- Bayrakci, M.; Gezici, O.; Zeki Bas, S.; Ozmen, M.; Maltas, E. Novel Humic Acid-Bonded Magnetite Nanoparticles for Protein Immobilization. Mater. Sci. Eng. C 2014, 42, 546–552. [Google Scholar] [CrossRef]
- Gezici, O.; Kara, H.; Ersoz, M.; Abali, Y. The Sorption Behavior of a Nickel-Insolubilized Humic Acid System in a Column Arrangement. J. Colloid Interface Sci. 2005, 292, 381–391. [Google Scholar] [CrossRef]
- Gezici, O.; Kara, H. Towards Multimodal HPLC Separations on Humic Acid-Bonded Aminopropyl Silica: RPLC and HILIC Behavior. Talanta 2011, 85, 1472–1482. [Google Scholar] [CrossRef]
- Yang, S.T.; Zong, P.F.; Ren, X.M.; Wang, Q.; Wang, X.K. Rapid and Highly Efficient Preconcentration of Eu(III) by Core–Shell Structured Fe3O4–Humic-Acid Magnetic Nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 6890–6899. [Google Scholar] [CrossRef]
- Durdureanu-Angheluta, A.; Dascalu, A.; Fifere, A.; Coroaba, A.; Pricop, L.; Chiriac, H.; Tura, V.; Pinteala, M.; Simionescu, B.C. Progress in the Synthesis and Characterization of Magnetite Nanoparticles with Amino Groups on the Surface. J. Magn. Magn. Mater. 2012, 324, 1679–1689. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, S.; Shao, Y.; Liu, J.; Xu, Z.; Zhu, D. Amino-functionalized Fe3O4@SiO2 Core–Shell Magnetic Nanomaterial as a Novel Adsorbent for Aqueous Heavy Metals Removal. J. Colloid Interface Sci. 2010, 349, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Bini, R.A.; Marques, R.F.C.; Santos, F.J.; Chaker, J.; Jafelissi, M., Jr. Synthesis and Functionalization of Magnetite Nanoparticles with Different Amino-Functional Alkoxysilanes. J. Magn. Magn. Mater. 2012, 324, 534–539. [Google Scholar] [CrossRef] [Green Version]
- Yamaura, M.; Camilo, R.L.; Sampaio, L.C.; Macedo, M.A.; Nakamura, M.; Toma, H.E. Preparation and Characterization of (3-Aminopropyl) Triethoxysilane-Coated Magnetite Nanoparticles. J. Magn. Magn. Mater. 2004, 279, 210–217. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Syhr, C.; Berensmeier, S. Controlled Synthesis of Magnetic Iron Oxide Nanoparticles: Magnetite Or Maghemite? Crystals 2020, 10, 214. [Google Scholar] [CrossRef] [Green Version]
- Stober, W.; Fink, A. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Ozmen, M.; Can, K.; Arslan, G.; Tor, A.; Cengeloglu, Y.; Ersoz, M. Adsorption of Cu(II) from Aqueous Solution by Using Modified Fe3O4 Magnetic Nano-Particles. Desalination 2010, 254, 162–169. [Google Scholar] [CrossRef]
- Gorski, C.A.; Scherer, M.M. Determination of Nanoparticulate Magnetite Stoichiometry by Mossbauer Spectroscopy, Acid Dissolution, and Powder X-Ray Diffraction: A Critical Review. Am. Mineral. 2010, 95, 1017–1026. [Google Scholar] [CrossRef]
- He, Y.T.; Traina, S.J. Transformation of Magnetite to Goethite under Alkaline pH Conditions. Clay Miner. 2007, 42, 13–19. [Google Scholar] [CrossRef]
- Kolhatkar, A.G.; Jamison, A.C.; Litvinov, D.; Willson, R.C.; Lee, T.R. Tuning the Magnetic Properties of Nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef] [Green Version]
- Roth, H.-C.; Schwaminger, S.P.; Schindler, M.; Wagner, F.E.; Berensmeier, S. Influencing Factors in The Co-precipitation Process of Superparamagnetic Iron Oxide Nanoparticles: A Model Based Study. J. Magn. Magn. Mater. 2015, 377, 81–89. [Google Scholar] [CrossRef]
- Ghorbani, F.; Kamari, S. Core–shell Magnetic Nanocomposite of Fe3O4@SiO2@NH2 as an Efficient and Highly Recyclable Adsorbent of Methyl Red Dye from Aqueous Environments. Environ. Technol. Innovat. 2019, 14, 100–113. [Google Scholar] [CrossRef]
- Romano, F.L.; Ambrosano, G.M.B.; de Araújo Magnani, M.B.B.; Nouer, D.F. Analysis of the coefficient of variation in shear and tensile bond strength tests. J. Appl. Oral Sci. 2005, 13, 243–246. [Google Scholar] [CrossRef]
- Namduri, H.; Nasrazadani, S. Quantitative Analysis of Iron Oxides using Fourier Transform Infrared Spectrophotometry. Corros. Sci. 2008, 50, 2493–2497. [Google Scholar] [CrossRef]
- Feng, B.; Hong, R.Y.; Wang, L.S.; Cuo, L.; Li, H.Z.; Ding, J.; Zheng, Y.; Wei, D.G. Synthesis of Fe3O4/APTES/PEG Di-Acid Functionalized Magnetic Nanoparticles for MR Imaging. Colloids Surf. A 2008, 328, 52–59. [Google Scholar] [CrossRef]
- Golub, A.A.; Zubenko, A.I.; Zhmud, B.V. γ-APTES Modified Silica Gels: The Structure of the Surface Layer. J. Colloid Interface Sci. 1996, 179, 482–487. [Google Scholar] [CrossRef]
- Dhavale, R.P.; Waifalkar, P.P.; Sharma, A.; Dhavale, R.P.; Sahoo, S.C.; Kollu, P.; Chougalle, A.D.; Zahn, D.R.T.; Salvan, G.; Patil, P.S.; et al. Monolayer Grafting of Aminosilane on Magnetic Nanoparticles: An Efficient Approach for Targeted Drug Delivery System. J. Colloid Interface Sci. 2018, 529, 415–425. [Google Scholar] [CrossRef]
- Dodi, G.; Hritcu, D.; Draganescu, D.; Andrei, R.D.; Popa, M.I. Hexagonal-shaped Aminosilane Magnetite Nanoparticles: Preparation, Characterization and Hybrid Film Deposition. Colloids Surf. A 2018, 542, 21–30. [Google Scholar] [CrossRef]
- White, L.D.; Tripp, C.P. Reaction of (3-Aminopropyl)dimethylethoxysilane with Amine Catalysts on Silica Surfaces. J. Colloid Interface Sci. 2000, 232, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Swathi, S.; Ameen, F.; Ravi, G.; Yuvakkumar, R.; Hong, S.I.; Velauthapillai, D.; Dang, C. Cancer Targeting Potential of Bioinspired Chain Like Magnetite (Fe3O4) Nanostructures. Curr. Appl. Physics 2020, 20, 982–987. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Q.; Finch, J.A. Silanation and Stability of 3-Aminopropyl Triethoxy Silane on Nanosized Superparamagnetic Particles: I. Direct Silanation. Appl. Surf. Sci. 1997, 120, 269–278. [Google Scholar] [CrossRef]
- Bruce, I.J.; Sen, T. Surface Modification of Magnetic Nanoparticles with Alkoxysilanes and their Application in Magnetic Bioseparations. Langmuir 2005, 21, 7029–7035. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; Wiley-VCH: Weinheim, Germany, 2003; 664р. [Google Scholar]
- Jolstere, R.; Gunneriusson, L.; Forsling, W.J. Surface Complexation Modeling of Fe3O4–H+ and Mg(II) Sorption onto Maghemite and Magnetite. J. Colloid Interface Sci. 2010, 386, 260–267. [Google Scholar] [CrossRef]
- Tombácz, E.; Illés, E.; Majzik, A.; Hajdú, A.; Rideg, N.; Szekeres, M. Ageing in the Inorganic Nanoworld: Example of Magnetite Nanoparticles in Aqueous Medium. Croat. Chem. Acta 2007, 80, 503–515. [Google Scholar]
- Zhu, C.-Y.; Li, Z.-Y.; Pan, N. Design and Thermal Insulation Performance Analysis of Endothermic Opacifiers Doped Silica Aerogels. Int. J. Thermal Sci. 2019, 145, 105995. [Google Scholar] [CrossRef]
- Illés, E.; Tombácz, E. The Role of Variable Surface Charge and Surface Complexation in the Adsorption of Humic Acid on Magnetite. Colloids Surf. A 2003, 230, 99–109. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chen, D.-H. Preparation and Adsorption Properties of Monodisperse Chitosan-Bound Fe3O4 Magnetic Nanoparticles for Removal of Cu(II) Ions. J. Colloid Interface Sci. 2005, 283, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.F.R.; Rocha, C.O.; Piazza, R.D.; dos Santos, C.C.; Morales, M.A.; Faria, F.S.E.D.V.; Marques, R.F.C. Synthesis, Characterization and Applications of Maghemite Beads Functionalized with Rabbit Antibodies. Nanotechnology 2018, 29, 365701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gdula, K.; Gładysz-Płaska, A.; Cristóvão, B.; Ferenc, W.; Skwarek, E. Amine-functionalized Magnetite-Silica Nanoparticles as Effective Adsorbent for Removal of Uranium(VI) Ions. J. Mol. Liq. 2019, 290, 111–125. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Linden, M. Towards Establishing Structure-Activity Relationships for Mesoporous Silica in Drug Delivery Applications. J. Control. Release 2008, 128, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Kralj, S.; Drofenik, M.; Makovec, D. Controlled Surface Functionalization of Silica-Coated Magnetic Nanoparticles with Terminal Amino and Carboxyl Groups. J. Nanoparticle Res. 2010, 13, 2829–2841. [Google Scholar] [CrossRef]
- Tombácz, E.; Tóth, I.Y.; Nesztor, D.; Illés, E.; Hajdú, A.; Szekeres, M.; Vékás, L. Adsorption of Organic Acids on Magnetite Nanoparticles, pH-dependent Colloidal Stability and Salt Tolerance. Colloids Surf. A 2013, 435, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Malfait, W.J.; Verel, R.; Koebel, M.M. Hydrophobization of Silica Aerogels: Insights From Quantitative Solid-State NMR Spectroscopy. J. Phys. Chem. C 2014, 118, 25545–25554. [Google Scholar] [CrossRef]
- Baalousha, M. Aggregation and disaggregation of iron oxide nanoparticles: Influence of Particle Concentration, pH and Natural Organic Matter. Sci. Total Environ. 2009, 407, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, A. The Supramolecular Structure of Humic Substances: A Novel Understanding of Humus Chemistry and Implications in Soil Science. Adv. Agron. 2002, 75, 57–134. [Google Scholar] [CrossRef]











| Sample | Fe3O4 | Fe3O4/APTES (Ar) | Fe3O4/APTES (Air) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| hkl | 2θ° | d, Å | FWHM | 2θ° | d, Å | FWHM | 2θ° | d, Å | FWHM |
| 220 | 45.45 | 2.965 | 0.636 (8) | 45.45 | 2.96 | 0.504 (9) | 45.65 | 2.956 | 0.721 (6) |
| 311 | 53.90 | 2.527 | 0.662 (2) | 53.95 | 2.53 | 0.679 (5) | 54.05 | 2.519 | 1.731 (2) |
| 400 | 66.30 | 2.095 | 0.780 (1) | 66.3 | 2.095 | 0.890 (4) | 66.4 | 2.089 | 0.822 (1) |
| 422 | 83.85 | 1.714 | 0.975 (2) | 84.05 | 1.712 | 0.844 (9) | 84.45 | 1.707 | 0.785 (1) |
| 511 | 90.70 | 1.610 | 0.940 (7) | 90.75 | 1.61 | 0.828 (5) | 90.7 | 1.609 | 1.891 (3) |
| 440 | 101.35 | 1.481 | 0.899 (1) | 101.37 | 1.481 | 1.026 (6) | 101.55 | 1.477 | 0.824 (4) |
| a, Å | 8.3813 | 8.3789 | 8.3603 | ||||||
| X | 0.37 | 0.35 | 0.187 | ||||||
| δ | 0.069 | 0.08 | 0.186 | ||||||
| Structure | Fe2.93O4 | Fe2.92O4 | Fe2.81O4 | ||||||
| % Fe3O4 | 78.8 | 75.8 | 42.4 | ||||||
| DXRD, nm | 17.1 ± 2.3 | 20.5 ± 3.3 | 16.5 ± 1.96 | ||||||
| CV, % | 13.5 | 16.1 | 9.5 | ||||||
| DSEM, nm | 32.1 ± 4.3 | 24.2 ± 2.8 | 23.3 ± 3.1 | ||||||
| CV, % | 13.5 | 11.6 | 10.5 | ||||||
| HA Amount | Fe3O4 | Fe3O4/APTES (Air) | Fe3O4/APTES (Ar) |
|---|---|---|---|
| for full neutralization of charge, g/g | 0.01 | 0.0085 | 0.005 |
| to reach −20 mV of zeta potential, g/g | 0.016 | 0.038 | 0.014 |
| to reach plateau, g/g | 0.04 | 0.056 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondarenko, L.; Illés, E.; Tombácz, E.; Dzhardimalieva, G.; Golubeva, N.; Tushavina, O.; Adachi, Y.; Kydralieva, K. Fabrication, Microstructure and Colloidal Stability of Humic Acids Loaded Fe3O4/APTES Nanosorbents for Environmental Applications. Nanomaterials 2021, 11, 1418. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11061418
Bondarenko L, Illés E, Tombácz E, Dzhardimalieva G, Golubeva N, Tushavina O, Adachi Y, Kydralieva K. Fabrication, Microstructure and Colloidal Stability of Humic Acids Loaded Fe3O4/APTES Nanosorbents for Environmental Applications. Nanomaterials. 2021; 11(6):1418. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11061418
Chicago/Turabian StyleBondarenko, Lyubov, Erzsébet Illés, Etelka Tombácz, Gulzhian Dzhardimalieva, Nina Golubeva, Olga Tushavina, Yasuhisa Adachi, and Kamila Kydralieva. 2021. "Fabrication, Microstructure and Colloidal Stability of Humic Acids Loaded Fe3O4/APTES Nanosorbents for Environmental Applications" Nanomaterials 11, no. 6: 1418. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11061418