Controlled Fabrication of Quality ZnO NWs/CNTs and ZnO NWs/Gr Heterostructures via Direct Two-Step CVD Method
Abstract
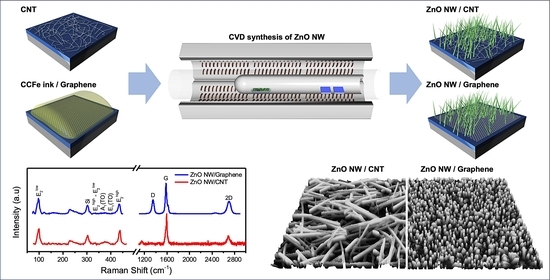
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Direct-Write Patterning (DWP) Method
2.3. CVD Synthesis Methods
2.3.1. CVD Synthesis of CNTs
2.3.2. CVD Synthesis of ZnO NWs
2.3.3. CVD Synthesis of Graphene
2.4. Measurements and Characterization Tools
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pitkethly, M.J. Nanometerials—The Driving Force. Mater. Today 2004, 7, 20–29. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-Shell Carbon Nanotubes of 1-nm Diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Bethune, D.S.; Kiang, C.H.; de Vries, M.S.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. Cobalt-Catalysed Growth of Carbon Nanotubes with Single-Atomic-Layer Walls. Nature 1993, 363, 605–607. [Google Scholar] [CrossRef]
- Ruddaraju, L.K.; Pammi, S.V.N.; Pallela, P.N.V.K.; Padavala, V.S.; Kolapalli, V.R.M. Antibiotic Potentiation and Anti-Cancer Competence through Bio-Mediated ZnO Nanoparticles. Mater. Sci. Eng. C 2019, 103, 109756. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of Photogenerated Reactive Oxygen Species and Correlation with the Antibacterial Properties of Engineered Metal-Oxide Nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Modugno, G.; Ménard-Moyon, C.; Prato, M.; Bianco, A. Carbon Nanomaterials Combined with Metal Nanoparticles for Theranostic Applications. Br. J. Pharm. 2015, 172, 975–991. [Google Scholar] [CrossRef]
- Pandurangan, M.; Enkhtaivan, G.; Kim, H.D. Anticancer Studies of Synthesized ZnO Nanoparticles against Human Cervical Carcinoma Cells. J. Photochem. Photobiol. B Biol. 2016, 158, 206–211. [Google Scholar] [CrossRef]
- Hanley, C.; Layne, J.; Punnoose, A.; Reddy, K.M.; Coombs, I.; Coombs, A.; Feris, K.; Wingett, D. Preferential Killing of Cancer Cells and Activated Human T Cells Using ZnO Nanoparticles. Nanotechnology 2008, 19, 295103. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc Oxide Nanoparticles for Selective Destruction of Tumor Cells and Potential for Drug Delivery Applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, N.; Fessi, H.; Elaissari, A. Theranostic Applications of Nanoparticles in Cancer. Drug Discov. 2012, 17, 928–934. [Google Scholar] [CrossRef]
- Wang, J.; Lee, J.S.; Kim, D.; Zhu, L. Exploration of Zinc Oxide Nanoparticles as a Multitarget and Multifunctional Anticancer Nanomedicine. ACS Appl. Mater. Interfaces 2017, 9, 39971–39984. [Google Scholar] [CrossRef]
- Keefer, E.W.; Botterman, B.R.; Romero, M.I.; Rossi, A.F.; Gross, G.W. Carbon Nanotube Coating Improves Neuronal Recordings. Nat. Nanotechnol. 2008, 3, 434–439. [Google Scholar] [CrossRef]
- Schwartz, A.B.; Cui, X.T.; Weber, D.J.; Moran, D.W. Brain-Controlled Interfaces: Movement Restoration with Neural Prosthetics. Neuron 2006, 52, 205–220. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Martini, N.; Hassler, C.; Kirch, R.D.; Stieglitz, T.; Seifert, A.; Hofmann, U.G. In Vivo Monitoring of Glial Scar Proliferation on Chronically Implanted Neural Electrodes by Fiber Optical Coherence Tomography. Front. Neuroeng. 2014, 7, 00034. [Google Scholar] [CrossRef] [PubMed]
- Kordzadeh, A.; Amjad-Iranagh, S.; Zarif, M.; Modarress, H. Adsorption and Encapsulation of the Drug Doxorubicin on Covalent Functionalized Carbon Nanotubes: A Scrutinized Study by Using Molecular Dynamics Simulation and Quantum Mechanics Calculation. J. Mol. Graph. Mod. 2019, 88, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Kocaman, S.; Gursoy, M.; Karaman, M.; Ahmetli, G. Synthesis and Plasma Surface Functionalization of Carbon Nanotubes for Using in Advanced Epoxy-Based Nanocomposites. Surf. Coat. Technol. 2020, 399, 126144. [Google Scholar] [CrossRef]
- Zhu, Y.; Elim, H.I.; Foo, Y.L.; Yu, T.; Liu, Y.; Ji, W.; Lee, J.Y.; Shen, Z.; Wee, A.T.S.; Thong, J.T.L.; et al. Multiwalled Carbon Nanotubes Beaded with ZnO Nanoparticles for Ultrafast Nonlinear Optical Switching. Adv. Mater. 2006, 18, 587–592. [Google Scholar] [CrossRef]
- Zhao, T.; She, S.; Ji, X.; Jin, W.; Dang, A.; Li, H.; Li, T.; Shang, S.; Zhou, Z. In-Situ Growth Amorphous Carbon Nanotube on Silicon Particles as Lithium-Ion Battery Anode Materials. J. Alloys Compd. 2017, 708, 500–507. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon Nanotubes-the Route toward Applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ok, J.G.; Tawfick, S.H.; Juggernauth, K.A.; Sun, K.; Zhang, Y.; Hart, A.J. Electrically Addressable Hybrid Architectures of Zinc Oxide Nanowires Grown on Aligned Carbon Nanotubes. Adv. Funct. Mater. 2010, 20, 2470–2480. [Google Scholar] [CrossRef] [Green Version]
- Caglar, M.; Ilican, S.; Caglar, Y.; Yakuphanoglu, F. Electrical Conductivity and Optical Properties of ZnO Nanostructured Thin Film. Appl. Surf. Sci. 2009, 255, 4491–4496. [Google Scholar] [CrossRef]
- Xu, F.; Qin, Q.; Mishra, A.; Gu, Y.; Zhu, Y. Mechanical Properties of ZnO Nanowires under Different Loading Modes. Nano Res. 2010, 3, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Bettini, S.; Pagano, R.; Bonfrate, V.; Maglie, E.; Manno, D.; Serra, A.; Valli, L.; Giancane, G. Promising Piezoelectric Properties of New ZnO@Octadecylamine Adduct. J. Phys. Chem. C 2015, 119, 20143–20149. [Google Scholar] [CrossRef]
- Ta, H.Q.; Zhao, L.; Pohl, D.; Pang, J.; Trzebicka, B.; Rellinghaus, B.; Pribat, D.; Gemming, T.; Liu, Z.; Bachmatiuk, A.; et al. Graphene-like ZnO: A Mini Review. Crystals 2016, 6, 100. [Google Scholar] [CrossRef] [Green Version]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphologies. Nanomaterials 2009, 10, 1086. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.K.; Shrestha, S.; Tiwari, A.P.; Kim, J.I.; Ko, S.W.; Kim, H.J.; Park, C.H.; Kim, C.S. Bio-Inspired Hybrid Scaffold of Zinc Oxide-Functionalized Multi-Wall Carbon Nanotubes Reinforced Polyurethane Nanofibers for Bone Tissue Engineering. Mater. Des. 2017, 133, 69–81. [Google Scholar] [CrossRef]
- Xie, J.; Frachioni, A.; Williams, D.S.; White, B.E. Thermal Conductivity of a ZnO Nanowire/Silica Aerogel Nanocomposite. Appl. Phys. Lett. 2013, 102, 193101. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Zhou, C. Review of Chemical Vapor Deposition of Graphene and Related Applications. Acc. Chem. Res. 2013, 46, 2329–2339. [Google Scholar] [CrossRef]
- Singh, L.T.; Sugavaneshwar, R.P.; Nanda, K.K. Carbon Nanotube-ZnO Nanowire Hybrid Architectures as Multifunctional Devices. AIP Adv. 2013, 3, 082106. [Google Scholar] [CrossRef]
- Song, Z.; Wei, H.; Liu, Y.; Wang, J.; Long, H.; Wang, H.; Qin, P.; Zeng, W.; Fang, G. Enhanced Field Emission from Aligned ZnO Nanowires Grown on a Graphene Layer with Hydrothermal Method. IEEE Trans. Nanotechnol. 2014, 13, 167–171. [Google Scholar] [CrossRef]
- Anand, K.; Singh, O.; Singh, M.P.; Kaur, J.; Singh, R.C. Hydrogen Sensor Based on Graphene/ZnO Nanocomposite. Sens. Actuators B Chem. 2014, 195, 409–415. [Google Scholar] [CrossRef]
- Panth, M.; Cook, B.; Zhang, Y.; Ewing, D.; Tramble, A.; Wilson, A.; Wu, J. High-Performance Strain Sensors Based on Vertically Aligned Piezoelectric Zinc Oxide Nanowire Array/Graphene Nanohybrids. ACS Appl. Nano Mater. 2020, 3, 6711–6718. [Google Scholar] [CrossRef]
- Nourmohammadi, A.; Rahighi, R.; Akhavan, O.; Moshfegh, A. Graphene Oxide Sheets Involved in Vertically Aligned Zinc Oxide Nanowires for Visible Light Photoinactivation of Bacteria. J. Alloys Compd. 2014, 612, 380–385. [Google Scholar] [CrossRef]
- Fu, X.W.; Liao, Z.M.; Zhou, Y.B.; Wu, H.C.; Bie, Y.Q.; Xu, J.; Yu, D.P. Graphene/ZnO Nanowire/Graphene Vertical Structure Based Fast-Response Ultraviolet Photodetector. Appl. Phys. Lett. 2012, 100, 223114. [Google Scholar] [CrossRef]
- Lee, J.M.; Pyun, Y.B.; Yi, J.; Choung, J.W.; Park, W.I. ZnO Nanorod-Graphene Hybrid Architectures for Multifunctional Conductors. J. Phys. Chem. C 2009, 113, 19134–19138. [Google Scholar] [CrossRef]
- Alameri, D.; Ocola, L.E.; Kuljanishvili, I. Mask-Free Fabrication and Chemical Vapor Deposition Synthesis of Ultrathin Zinc Oxide Microribbons on Si/SiO2 and 2D Substrates. J. Vac. Sci. Technol. A 2018, 36, 05G506. [Google Scholar] [CrossRef]
- Alameri, D.; Ocola, L.E.; Kuljanshvili, I. Controlled Selective CVD Growth of ZnO Nanowires Enabled by Mask-Free Fabrication Approach Using Aqueous Fe Catalytic Inks. Adv. Mater. Interfaces 2017, 4, 1700950. [Google Scholar] [CrossRef]
- Al-Asadi, A.S.; Henley, L.A.; Wasala, M.; Muchharla, B.; Perea-Lopez, N.; Carozo, V.; Lin, Z.; Terrones, M.; Mondal, K.; Kordas, K.; et al. Aligned Carbon Nanotube/Zinc Oxide Nanowire Hybrids as High Performance Electrodes for Supercapacitor Applications. J. Appl. Phys. 2017, 121, 124303. [Google Scholar] [CrossRef]
- Geng, C.; Jiang, Y.; Yao, Y.; Meng, X.; Zapien, J.A.; Lee, C.S.; Lifshitz, Y.; Lee, S.T. Well-Aligned ZnO Nanowire Arrays Fabricated on Silicon Substrates. Adv. Funct. Mater. 2004, 14, 589–594. [Google Scholar] [CrossRef]
- Little, D.J.; Pfund, J.D.; Mclain, A.A.; Sporie, J.A.; Lantvit, S.M.; King, S.T. Synthesis of a Zinc Oxide/Graphene Hybrid Material by the Direct Thermal Decomposition of Oxalate. Mater. Res. Express 2020, 7, 65005. [Google Scholar] [CrossRef]
- Kuljanishvili, I.; Dikin, D.A.; Rozhok, S.; Mayle, S.; Chandrasekhar, V. Controllable Patterning and CVD Growth of Isolated Carbon Nanotubes with Direct Parallel Writing of Catalyst Using Dip Pen Nanolithography. Small 2009, 5, 2523–2527. [Google Scholar] [CrossRef] [Green Version]
- Dong, R.; Moore, L.; Ocola, L.E.; Kuljanishvili, I. Enabling Quality Interfaces with Mask-Free Approach to Selective Growth of MoS2/Graphene Stacked Structures. Adv. Mater. Interfaces 2016, 3, 1600098. [Google Scholar] [CrossRef]
- Wongchoosuk, C.; Subannajui, K.; Menzel, A.; Burshtein, I.A.; Tamir, S.; Lifshitz, Y.; Zacharias, M. Controlled Synthesis of ZnO Nanostructures: The Role of Source and Substrate Temperatures. J. Phys. Chem. C 2011, 115, 757–761. [Google Scholar] [CrossRef]
- Jorio, A.; Pimenta, M.A.; Souza Filho, A.G.; Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Characterizing Carbon Nanotube Samples with Resonance Raman Scattering. New J. Phys. 2003, 5, 139. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Souza Filho, A.G.; Saito, R. Defect Characterization in Graphene and Carbon Nanotubes Using Raman Spectroscopy. Philos. Trans. R. Soc. 2010, 368, 5355–5377. [Google Scholar] [CrossRef]
- Arguello, C.A.; Rousseau, D.L.; Porto, S.P.S.; Reynolds, D.C.; Collins, T.C.; Bauman, R.P. First-Order Raman Effects in Wurtzite-Type Crystals. Phys. Rev. 1969, 181, 1351–1363. [Google Scholar] [CrossRef]
- Cheng, A.J.; Tzeng, Y.; Xu, H.; Alur, S.; Wang, Y.; Park, M.; Wu, T.H.; Shannon, C.; Kim, D.J.; Wang, D. Raman Analysis of Longitudinal Optical Phonon-Plasmon Coupled Modes of Aligned ZnO Nanorods. J. Appl. Phys. 2009, 105, 073104. [Google Scholar] [CrossRef] [Green Version]
- Calzolari, A.; Nardelli, M.B. Dielectric Properties and Raman Spectra of ZnO from a First Principles Finite-Differences/Finite-Fields Approach. Sci. Rep. 2013, 3, 2999. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Li, X.; Zhan, Z.; Wang, L.; Feng, S.; Chai, X.; Lu, W.; Shen, J.; Weng, Z.; Sun, J. Catalyst-Free, Selective Growth of ZnO Nanowires on SiO2 by Chemical Vapor Deposition for Transfer-Free Fabrication of UV Photodetectors. ACS Appl. Mater. Interfaces 2015, 7, 20264–20271. [Google Scholar] [CrossRef]
- Zhu, G.; Zhou, Y.; Wang, S.; Yang, R.; Ding, Y.; Wang, X.; Bando, Y.; Wang, Z.L. Synthesis of Vertically Aligned Ultra-Long ZnO Nanowires on Heterogeneous Substrates with Catalyst at the Root. Nanotechnology 2012, 23, 055604. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chen, T.-L.; Gu, Y.; Warren, J.; Osgood, R.M. Zinc Oxide Nanowires Grown by Vapor-Phase Transport Using Selected Metal Catalysts: A Comparative Study. Chem. Mater. 2005, 17, 4227–4234. [Google Scholar] [CrossRef]
- Lim, K.; Hamid, M.A.A.; Shamsudin, R.; Al-Hardan, N.H.; Mansor, I.; Chiu, W. Temperature-Driven Structural and Morphological Evolution of Zinc Oxide Nano-Coalesced Microstructures and Its Defect-Related Photoluminescence Properties. Materials 2016, 9, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.M.; Shima, H.; Son, S.; Hahn, J.R. In Situ Fabrication of a Thermally Stable and Highly Porous Conductive Solar Light-Driven ZnO-CNT Fiber Photocatalyst. RSC Adv. 2016, 6, 71450–71460. [Google Scholar] [CrossRef]
- Sharma, M.; Joshi, M.; Nigam, S.; Shree, S.; Avasthi, D.K.; Adelung, R.; Srivastava, S.K.; Kumar Mishra, Y. ZnO Tetrapods and Activated Carbon Based Hybrid Composite: Adsorbents for Enhanced Decontamination of Hexavalent Chromium from Aqueous Solution. Chem. Eng. J. 2019, 358, 540–551. [Google Scholar] [CrossRef]
- Cao, A.; Ajayan, P.M.; Ramanath, G.; Baskaran, R.; Turner, K. Silicon Oxide Thickness-Dependent Growth of Carbon Nanotubes. Appl. Phys. Lett. 2004, 84, 109–111. [Google Scholar] [CrossRef] [Green Version]
- Simmons, J.M.; Nichols, B.M.; Marcus, M.S.; Castellini, O.M.; Hamers, R.J.; Eriksson, M.A. Critical Oxide Thickness for Efficient Single-Walled Carbon Nanotube Growth on Silicon Using Thin SiO2 Diffusion Barriers. Small 2006, 2, 902–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.J.; Wei; Vajtai, R.; Ajayan, P.M.; Homma, Y.; Prabhakaran, K.; Ogino, T. Mechanism of Selective Growth of Carbon Nanotubes on SiO2/Si Patterns. Nano Lett. 2003, 3, 561–564. [Google Scholar] [CrossRef]
- Xiang, R.; Einarsson, E.; Okabe, H.; Chiashi, S.; Shiomi, J.; Maruyama, S. Patterned Growth of High-Quality Single-Walled Carbon Nanotubes from Dip-Coated Catalyst. Jpn. J. Appl. Phys. 2010, 49, 02BA03. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaper, N.; Alameri, D.; Kim, Y.; Thomas, B.; McCormack, K.; Chan, M.; Divan, R.; Gosztola, D.J.; Liu, Y.; Kuljanishvili, I. Controlled Fabrication of Quality ZnO NWs/CNTs and ZnO NWs/Gr Heterostructures via Direct Two-Step CVD Method. Nanomaterials 2021, 11, 1836. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11071836
Schaper N, Alameri D, Kim Y, Thomas B, McCormack K, Chan M, Divan R, Gosztola DJ, Liu Y, Kuljanishvili I. Controlled Fabrication of Quality ZnO NWs/CNTs and ZnO NWs/Gr Heterostructures via Direct Two-Step CVD Method. Nanomaterials. 2021; 11(7):1836. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11071836
Chicago/Turabian StyleSchaper, Nicholas, Dheyaa Alameri, Yoosuk Kim, Brian Thomas, Keith McCormack, Mathew Chan, Ralu Divan, David J. Gosztola, Yuzi Liu, and Irma Kuljanishvili. 2021. "Controlled Fabrication of Quality ZnO NWs/CNTs and ZnO NWs/Gr Heterostructures via Direct Two-Step CVD Method" Nanomaterials 11, no. 7: 1836. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11071836