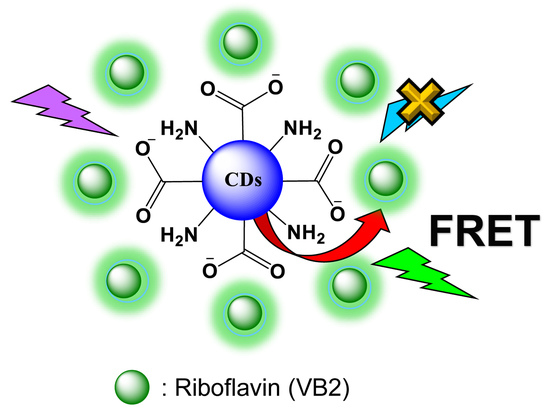
Optimizing the Efficiency of a Cytocompatible Carbon-Dots-Based FRET Platform and Its Application as a Riboflavin Sensor in Beverages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Preparation of Carbon Dots (CDs)
2.3. Investigation of the CDs’ Stability
2.4. FRET Assays between CDs and RF
2.4.1. Selection of the Donor Excitation Wavelength
2.4.2. Selection of the Donor Concentration
2.4.3. Calculation of FRET Parameters
2.5. Interference and Competition Analysis
2.6. Cell Culture
2.7. Cytocompatibility Test
2.8. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of CDs
3.2. Optimization of the FRET Process
3.2.1. Selection of pH
3.2.2. Selection of the Excitation Wavelength
3.2.3. Influence of Donor Concentration
3.3. Calibration Curve
3.4. Selectivity of CDs–RF Conjugates for the RF Detection
3.5. Comparison with Other Methods
3.6. The Fluorescent Ratiometric Detection of RF in Real Samples
3.7. Preliminary Tests of Cytocompatibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esteves da Silva, J.C.G.; Goncalves, H.M.R. Analytical and bioanalytical applications of carbon dots. Trends Anal. Chem. 2011, 30, 1327–1336. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Tang, J.; Kang, B.; Wu, H.; Xu, M.; Wang, Y.; Zheng, G. Carbon nanodots featuring efficient FRET for real-time monitoring of drug delivery and two-photon imaging. Adv. Mater. 2013, 25, 6569–6574. [Google Scholar] [CrossRef] [PubMed]
- Jana, J.; Aditya, T.; Pal, T. Carbon dot–Au(I)Ag(0) assembly for the construction of an artificial light harvesting system. Dalton Trans. 2018, 47, 3580–3587. [Google Scholar] [CrossRef]
- Kundu, S.; Patra, A. Nanoscale Strategies for Light Harvesting. Chem. Rev. 2017, 117, 712–757. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.B.; Kim, Y.P. Analysis of protease activity using quantum dots and resonance energy transfer. Theranostics 2012, 2, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Lei, Y. Fluorescent carbon dots and their sensing applications. Trends Anal. Chem. 2017, 89, 163–180. [Google Scholar] [CrossRef]
- Lanvaya, N.; Radhakrishnan, S.; Sekar, C.; Navaneethanb, M.; Hayakawab, Y. Fabrication of Cr doped SnO2 nanoparticles based biosensor for the selective determination of riboflavin in pharmaceuticals. Analyst 2013, 138, 2061–2067. [Google Scholar]
- Wang, P.; Yin, Y.; Eremin, S.A.; Rybakov, V.B.; Zhang, T.; Xu, Z.; Ren, L.; He, X.; Meng, M.; Xi, R. Indirect competitive immunoassay for detection of vitamin B2 in foods and pharmaceuticals. J. Agric. Food Chem. 2013, 61, 7048–7054. [Google Scholar] [CrossRef]
- Saedisomeolia, A.; Ashoori, M. Chapter Two—Riboflavin in human health: A review of current evidences. Adv. Food Nutr. Res. 2018, 83, 57–81. [Google Scholar]
- Bartzatt, R.; Wol, T. Detection and assay of vitamin B-2 (riboflavin) in alkaline borate buffer with UV/Visible spectrophotometry. Int. Sch. Res. Not. 2014, 2014, 453085. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A.M.; Fernandez, C.; Whitehead, R.D., Jr.; Morales-A, P.; Boyd Barr, D.; Wilder, L.C.; Baker, S.E. Quantification of riboflavin in human urine using high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. B. 2011, 879, 1823–1826. [Google Scholar] [CrossRef]
- Sá, E.S.; Da Silva, P.S.; Jost, C.L.; Spinelli, A. Electrochemical sensor based on bismuth-film electrode for voltammetric studies on vitamin B2 (riboflavin). Sens. Actuators B Chem. 2015, 209, 423–430. [Google Scholar] [CrossRef]
- Madhuvilakku, R.; Alagar, S.; Mariappan, R.; Piraman, S. Green one-pot synthesis of flowers-like Fe3O4/rGO hybrid nanocomposites for effective electrochemical detection of riboflavin and low-cost supercapacitor applications. Sens. Actuators B 2017, 253, 879–892. [Google Scholar] [CrossRef]
- Chen, J.; Sun, J.; Liu, S. Determination of riboflavin in cereal grains by capillary electrophoresis with laser-induced fluorescence detection with on-line concentration. Anal. Lett. 2013, 46, 887–899. [Google Scholar] [CrossRef]
- Han, J.; Zou, H.Y.; Gao, M.X.; Huang, C.Z. A graphitic carbon nitride based fluorescence resonance energy transfer detection of riboflavin. Talanta 2016, 148, 279–284. [Google Scholar] [CrossRef]
- Lin, L.; Wang, Y.; Xiao, Y.; Chen, X. Ratiometric fluorescence detection of riboflavin based on fluorescence resonance energy transfer from nitrogen and phosphorus co-doped carbon dots to riboflavin. Anal. Bioanal. Chem. 2019, 411, 2803–2808. [Google Scholar] [CrossRef]
- Feng, S.; Pei, F.; Wua, Y.; Lv, J.; Hao, Q.; Yang, T.; Tong, Z.; Lei, W. A ratiometric fluorescent sensor based on g-CNQDs@Zn-MOF for the sensitive detection of riboflavin via FRET. Spectrochim. Acta Part A 2021, 246, 119004. [Google Scholar] [CrossRef]
- Wang, J.; Su, S.; Wei, J.; Bahgi, R.; Hope-Weeks, L.; Qiu, J.; Wang, S. Ratiometric sensor to detect riboflavin via fluorescence resonance energy transfer with ultrahigh sensitivity. Phys. E 2015, 72, 17–24. [Google Scholar] [CrossRef]
- Scholes, G.D.; Andrews, D.L. Resonance energy transfer and quantum dots. Phys. Rev. B 2005, 72, 125331. [Google Scholar] [CrossRef]
- Medintz, I.L.; Mattoussi, H. Quantum dot-based resonance energy transfer and its growing application in biology. Phys. Chem. Chem. Phys. 2009, 11, 17–45. [Google Scholar] [CrossRef]
- Sebrell, W.H.; Harris, R.S. The Vitamins: Chemistry, Physiology, Pathology, Methods, 2nd ed.; Academic Press: London, UK, 1972. [Google Scholar]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. 2013, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Lu, J.; Yan, M.; Yu, F.; Yu, J.; Sun, X. Fluorescence resonance energy transfer sensor between quantum dot donors and neutral red acceptors and its detection of BSA in micelles. Dye. Pigm. 2011, 91, 304–308. [Google Scholar] [CrossRef]
- Topete, A.; Melgar, D.; Alatorre-Meda, M.; Iglesias, P.; Argibay, B.; Vidawati, S.; Barbosa, S.; Costoya, J.A.; Taboada, P.; Mosquera, V. NIR-light active hybrid nanoparticles for combined imaging and bimodal therapy of cancerous cells. J. Mat. Chem. B 2014, 2, 6967–6977. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, S.; Topete, A.; Alatorre-Meda, M.; Villar-Alvarez, E.M.; Pardo, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Taboada, P.; Mosquera, V. Targeted combinatorial therapy using gold nanostars as theranostic platforms. J. Phys. Chem. C 2014, 118, 26313–26323. [Google Scholar] [CrossRef]
- Topete, A.; Alatorre-Meda, M.; Villar-Alvarez, E.M.; Carregal-Romero, S.; Barbosa, S.; Parak, W.J.; Taboada, P.; Mosquera, V. Polymeric-gold nanohybrids for combined imaging and cancer therapy. Adv. Healthc. Mater. 2014, 3, 1309–1325. [Google Scholar] [CrossRef]
- Rodríguez-Velázquez, E.; Silva, M.; Taboada, P.; Mano, J.F.; Suárez-Quintanilla, D.; Alatorre-Meda, M. Enhanced cell affinity of chitosan membranes mediated by superficial cross-linking: A straightforward method attainable by standard laboratory procedures. Biomacromolecules 2014, 15, 291–301. [Google Scholar] [CrossRef]
- Shi, L.; Li, Y.; Li, X.; Wen, X.; Zhang, G.; Yang, J.; Donga, C.; Shuang, S. Facile and eco-friendly synthesis of green fluorescent carbon nanodots for applications in bioimaging, patterning and staining. Nanoscale 2015, 7, 7394–7401. [Google Scholar] [CrossRef]
- Ding, H.; Yu, S.-B.; Wei, J.-S.; Xiong, H.-M. Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano 2016, 10, 484–491. [Google Scholar] [CrossRef]
- Clapp, A.R.; Medintz, I.L.; Mauro, J.M.; Fisher, B.R.; Bawendi, M.G.; Mattoussi, H. Fluorescence resonance energy transfer between quantum dot donors and dye-labeled protein acceptors. J. Am. Chem. Soc. 2004, 126, 301–310. [Google Scholar] [CrossRef]
- Lunz, M.; Bradley, A.L. Concentration dependence of Förster resonant energy transfer between donor and acceptor nanocrystal quantum dot layers: Effect of donor-donor interactions. Phys. Rev. B 2011, 83, 115423. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006; pp. 443–472. [Google Scholar]
- Aberásturi, F.J.; Jiménez, A.I.; Arias, J.J.; Jiménez, F. Simultaneous spectrophotometric determination of folic acid, pyridoxine, riboflavin, and thiamine by partial least-squares regression. Anal. Lett. 2002, 35, 1677–1691. [Google Scholar] [CrossRef]
- García, L.; Blázquez, S.; San Andrés, M.P.; Vera, S. Determination of thiamine, riboflavin and pyridoxine in pharmaceuticals by synchronous fluorescence spectrometry in organized media. Anal. Chim. Acta 2001, 434, 193–199. [Google Scholar] [CrossRef]
- Ma, Q.; Song, J.; Zhang, S.; Wang, M.; Guo, Y.; Dong, C. Colorimetric detection of riboflavin by silver nanoparticles capped with β-cyclodextrin-grafted citrate. Colloids Surf. B 2016, 148, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Nandi, S.; Layek, R.K.; Nandi, A.K. Fluorescence resonance energy transfer from sulfonated graphene to riboflavin: A simple way to detect vitamin B2. ACS Appl. Mater. Interfaces 2013, 5, 7392–7399. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; García-García, D.; San Andrés, M.P.; Vera, S. Determination of riboflavin based on fluorescence quenching by graphene dispersions in polyethylene glycol. RSC Adv. 2016, 6, 19686–19699. [Google Scholar] [CrossRef]
- Osorio, M.V.; Márques, S.S.; Oliveira, H.M.; Barreiros, L.; Segundo, M.A. Fluorometric method based on molecular recognition solid-phase extraction for determination of riboflavin in milk and infant formula. J. Food Compos. Anal. 2016, 45, 141–146. [Google Scholar] [CrossRef]
- Mattivi, F.; Monetti, A.; Vrhovšek, U.; Tonon, D.; Andrés-Lacueva, C. High-performance liquid chromatographic determination of the riboflavin concentration in white wines for predicting their resistance to light. J. Chromatogr. A 2000, 888, 121–127. [Google Scholar] [CrossRef]
- Zougagh, M.; Ríos, A. Supercritical fluid extraction as an on-line clean-up technique for determination of riboflavin vitamins in food samples by capillary electrophoresis with fluorimetric detection. Electrophoresis 2008, 29, 3213–3219. [Google Scholar] [CrossRef]
- Li, H.; Shao, F.-Q.; Zou, S.-Y.; Yang, Q.-J.; Huang, H.; Feng, J.-J.; Wang, A.-J. Microwave-assisted synthesis of N,P-doped carbon dots for fluorescent cell imaging. Microchim. Acta 2016, 183, 821–826. [Google Scholar] [CrossRef]
- Zhang, Z.; Duan, Y.; Yu, Y.; Yan, Z.; Chen, J. Carbon quantum dots: Synthesis, characterization, and assessment of cytocompatibility. J. Mater. Sci. Mater. Med. 2015, 26, 213. [Google Scholar] [CrossRef] [PubMed]
- Alatorre-Meda, M.; Taboada, P.; Hartl, F.; Wagner, T.; Freis, M.; Rodríguez, J.R. The influence of chitosan valence on the complexation and transfection of DNA: The weaker the DNA–chitosan binding the higher the transfection efficiency. Colloids Surf. B 2011, 82, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Alatorre-Meda, M.; Taboada, P.; Krajewska, B.; Willemeit, M.; Deml, A.; Klösel, R.; Rodríguez, J.R. DNA−poly (diallyldimethylammonium chloride) complexation and transfection efficiency. J. Phys. Chem. B 2010, 114, 9356–9366. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Herrera, D.; Rodríguez-Velázquez, E.; Alatorre-Meda, M.; Paraguay-Delgado, F.; Tirado-Guízar, A.; Taboada, P.; Pina-Luis, G. NIR-emitting alloyed CdTeSe QDs and organic dye assemblies: A nontoxic, stable, and efficient FRET system. Nanomaterials 2018, 8, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xian, W. A Laboratory Course in Biomaterials; CRC Press: Boca Raton, FL, USA, 2010; p. 115. [Google Scholar]
- Assad, M.; Jackson, N. Biocompatibility evaluation of orthopedic biomaterials and medical devices: A review of safety and efficacy models. In Encyclopedia of Biomedical Engineering; Narayan, R., Ed.; Elsevier: Oxford, UK, 2019; pp. 281–309. [Google Scholar]
- Piluso, S.; Flores Gomez, D.; Dokter, I.; Moreira Texeira, L.; Li, Y.; Leijten, J.; van Weeren, R.; Vermonden, T.; Karperien, M.; Malda, J. Rapid and cytocompatible cell-laden silk hydrogel formation via riboflavin-mediated crosslinking. J. Mat. Chem. B 2020, 8, 9566–9575. [Google Scholar] [CrossRef]
- Ferri, G.; Digiacomo, L.; D’Autilia, F.; Durso, W.; Caracciolo, G.; Cardarelli, F. Time-lapse confocal imaging datasets to assess structural and dynamic properties of subcellular nanostructures. Sci. Data 2018, 5, 180191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phelps, M.A.; Foraker, A.B.; Gao, W.; Dalton, J.T.; Swaan, P.W. A novel rhodamine-riboflavin conjugate probe exhibits distinct fluorescence resonance energy transfer that enables riboflavin trafficking and subcellular localization studies. Mol. Pharm. 2004, 1, 257–266. [Google Scholar] [CrossRef]








| FRET Parameters | |||||
|---|---|---|---|---|---|
| Conjugate | J (cm3 L mol−1) | E (%) | R0 (nm) | r (nm) | K (M−1) |
| CDs/RF | 3.3 × 10−15 | 91 | 3.3 | 1.8 | 2.7 × 104 |
| Method/Sensor Material/Detection | Linear Range, µM | Det. Limit, µM | Ref |
|---|---|---|---|
| Cr-doped SnO2 nanoparticles electrode/Cyclic Voltammetry | 0.2–100 | 0.107 | [8] |
| Bismuth-film electrode/Cyclic Voltammetry | 0.3–0.8 | 0.1 | [13] |
| Fe3O4/Graphene oxide electrode/Differential pulse voltammetry | 0.3–1.0 | 0.089 | [14] |
| Capillary Electrophoresis/Laser-Induced Fluorescence | 0.001–1.33 | 0.008 | [15] |
| HPLC/tandem mass spectrometry | 0.011–53.1 | 0.029 | [12] |
| Ag nanoparticles modified with β-CD/Colorimetry | 0.16–4.66 | 0.167 | [36] |
| Spectrophotometry/Absorbance at 440 nm | 0.797–123 | 0.79 | [11] |
| Graphitic carbon nitride/Fluorimetry (FRET) | 0.4–10 | 0.170 | [16] |
| Carbon-dot-codoped N, P/Fluorimetry (FRET) | 0.5–50 | 0.170 | [17] |
| g-CNQDs@Zn-MOF/Fluorimetry (FRET) | 0–1 | 0.015 | [18] |
| Sulfonated graphene/Fluorimetry (FRET) | 0.4–10 | 1.6 | [37] |
| N,S-doped carbon dots/Fluorimetry (FRET) | 0.56–7.4 | 1.9 | [19] |
| Graphene-PEG/Fluorescence Quenching | 0–6.3 | 60 | [38] |
| Molecularly Imprinted Polymer (PIM)/Fluorimetry | Not reported | 79.7 | [39] |
| Carbon dots (N, COO−)/Fluorimetry (FRET) | 0–11 | 0.025 | This work |
| Sample | Added (µM) | Found (µM) | Recovered (µM) | Recovered (%) | SD | RSD |
|---|---|---|---|---|---|---|
| Monster Energy | 2.5 | 3.81 | 6.46 | 106.30 | 0.02 | 3.64 |
| 3.5 | 7.21 | 97.26 | 0.01 | 1.13 | ||
| 4.5 | 8.38 | 101.60 | 0.02 | 2.67 | ||
| 5.5 | 9.22 | 98.37 | 0.03 | 3.40 | ||
| 6.5 | 1.01 | 97.59 | 0.01 | 0.75 | ||
| 7.5 | 1.16 | 104.50 | 0.02 | 1.28 | ||
| 8.5 | 1.20 | 96.58 | 0.01 | 0.69 | ||
| Green tea | 2.5 | 1.33 | 3.94 | 104.50 | 0.02 | 3.07 |
| 3.5 | 4.76 | 97.95 | 0.01 | 1.62 | ||
| 4.5 | 5.81 | 99.51 | 0.01 | 1.74 | ||
| 5.5 | 7.07 | 104.30 | 0.01 | 1.12 | ||
| 6.5 | 7.96 | 101.90 | 0.02 | 1.56 | ||
| 7.5 | 8.84 | 101.10 | 0.04 | 3.13 | ||
| 8.5 | 9.68 | 98.24 | 0.02 | 1.42 | ||
| White wine | 2.5 | 1.61 | 4.08 | 98.80 | 0.01 | 1.30 |
| 3.5 | 5.05 | 98.32 | 0.01 | 1.37 | ||
| 4.5 | 6.18 | 101.60 | 0.02 | 1.39 | ||
| 5.5 | 7.21 | 101.80 | 0.03 | 1.79 | ||
| 6.5 | 8.33 | 103.50 | 0.02 | 1.48 | ||
| 7.5 | 8.97 | 98.23 | 0.03 | 1.49 | ||
| 8.5 | 1.00 | 99.06 | 0.07 | 3.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotolongo-García, R.; Rodríguez-Velázquez, E.; Alatorre-Meda, M.; Oropeza-Guzmán, M.T.; Tirado-Guízar, A.; Pina-Luis, G. Optimizing the Efficiency of a Cytocompatible Carbon-Dots-Based FRET Platform and Its Application as a Riboflavin Sensor in Beverages. Nanomaterials 2021, 11, 1981. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11081981
Sotolongo-García R, Rodríguez-Velázquez E, Alatorre-Meda M, Oropeza-Guzmán MT, Tirado-Guízar A, Pina-Luis G. Optimizing the Efficiency of a Cytocompatible Carbon-Dots-Based FRET Platform and Its Application as a Riboflavin Sensor in Beverages. Nanomaterials. 2021; 11(8):1981. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11081981
Chicago/Turabian StyleSotolongo-García, Roberto, Eustolia Rodríguez-Velázquez, Manuel Alatorre-Meda, Mercedes T. Oropeza-Guzmán, Antonio Tirado-Guízar, and Georgina Pina-Luis. 2021. "Optimizing the Efficiency of a Cytocompatible Carbon-Dots-Based FRET Platform and Its Application as a Riboflavin Sensor in Beverages" Nanomaterials 11, no. 8: 1981. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11081981