Resistive Response of Carbon Nanotube-Based Composites Subjected to Water Aging
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
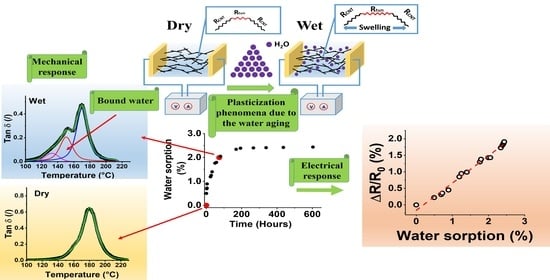
3.1. Electrical, Mechanical, and Water Absorption Properties
3.1.1. Electrical Properties
3.1.2. Mechanical and Water Absorption Properties
3.2. Thermomechanical and Water Diffusion Analysis (Water Aging Effect)
3.3. Resistive Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmadi, Z. Epoxy in nanotechnology: A short review. Prog. Org. Coat. 2019, 132, 445–448. [Google Scholar] [CrossRef]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Vertuccio, L.; Foglia, F.; Pantani, R.; Romero-Sánchez, M.; Calderón, B.; Guadagno, L. Carbon nanotubes and expanded graphite based bulk nanocomposites for de-icing applications. Compos. Part B Eng. 2020, 207, 108583. [Google Scholar] [CrossRef]
- Ou, B.; Wang, Y.; Lu, Y. A review on fundamentals and strategy of epoxy-resin-based anticorrosive coating materials. Polym. Technol. Mater. 2020, 60, 601–625. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Raimondo, M.; Barra, G.; Vertuccio, L.; Russo, S.; Lafdi, K.; Tucci, V.; Spinelli, G.; Lamberti, P. Influence of carbon nanoparticles/epoxy matrix interaction on mechanical, electrical and transport properties of structural advanced materials. Nanotechnology 2017, 28, 094001. [Google Scholar] [CrossRef]
- Vertuccio, L.; Sorrentino, A.; Guadagno, L.; Bugatti, V.; Raimondo, M.; Naddeo, C.; Vittoria, V. Behavior of epoxy composite resins in environments at high moisture content. J. Polym. Res. 2013, 20, 178. [Google Scholar] [CrossRef]
- Bao, L.-R.; Yee, A.F.; Lee, C.Y.-C. Moisture absorption and hygrothermal aging in a bismaleimide resin. Polymer 2001, 42, 7327–7333. [Google Scholar] [CrossRef]
- Glaskova, T.I.; Guedes, R.M.; Morais, J.J.; Aniskevich, A.N. A comparative analysis of moisture transport models as applied to an epoxy binder. Mech. Compos. Mater. 2007, 43, 377–388. [Google Scholar] [CrossRef]
- Alamri, H.; Low, I.M. Effect of water absorption on the mechanical properties of nano-filler reinforced epoxy nanocomposites. Mater. Des. 2012, 42, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Guadagno, L.; Vertuccio, L.; Sorrentino, A.; Raimondo, M.; Naddeo, C.; Vittoria, V.; Iannuzzo, G.; Calvi, E.; Russo, S. Mechanical and barrier properties of epoxy resin filled with multi-walled carbon nanotubes. Carbon 2009, 47, 2419–2430. [Google Scholar] [CrossRef]
- Starkova, O.; Buschhorn, S.; Mannov, E.; Schulte, K.; Aniskevich, A. Water transport in epoxy/MWCNT composites. Eur. Polym. J. 2013, 49, 2138–2148. [Google Scholar] [CrossRef]
- Barra, G.; Vertuccio, L.; Vietri, U.; Naddeo, C.; Hadavinia, H.; Guadagno, L. Toughening of epoxy adhesives by combined interaction of carbon nanotubes and silsesquioxanes. Materials 2017, 10, 1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guadagno, L.; Vertuccio, L.; Naddeo, C.; Calabrese, E.; Barra, G.; Raimondo, M.; Sorrentino, A.; Binder, W.; Michael, P.; Rana, S. Self-healing epoxy nanocomposites via reversible hydrogen bonding. Compos. Part B Eng. 2018, 157, 1–13. [Google Scholar] [CrossRef]
- Naddeo, C.; Vertuccio, L.; Barra, G.; Guadagno, L. Nano-charged polypropylene application: Realistic perspectives for enhancing durability. Materials 2017, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Vertuccio, L.; Guadagno, L.; Spinelli, G.; Russo, S.; Iannuzzo, G. Effect of carbon nanotube and functionalized liquid rubber on mechanical and electrical properties of epoxy adhesives for aircraft structures. Compos. Part B Eng. 2017, 129, 1–10. [Google Scholar] [CrossRef]
- Liu, M.; Luo, Y.; Jia, D. Facile synthesis of composite films featuring bulk superhydrophobicity, durability, and repairability for aquatic show. Compos. Sci. Technol. 2020, 197, 108231. [Google Scholar] [CrossRef]
- Liu, M.; Luo, Y.; Jia, D. A robust and versatile continuous super-repellent polymeric film for easy repair and underwater display. ACS Appl. Mater. Interfaces 2020, 12, 6677–6687. [Google Scholar] [CrossRef]
- Naddeo, C.; Guadagno, L.; De Luca, S.; Vittoria, V.; Camino, G. Mechanical and transport properties of irradiated linear low density polyethylene (LLDPE). Polym. Degrad. Stab. 2001, 72, 239–247. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Bidmeshkipour, S.; Abdi, Y.; Arzi, E.; Mohajerzadeh, S. Graphene based strain sensors: A comparative study on graphene and its derivatives. Appl. Surf. Sci. 2018, 448, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Ku-Herrera, J.; Avilés, F. Cyclic tension and compression piezoresistivity of carbon nanotube/vinyl ester composites in the elastic and plastic regimes. Carbon 2012, 50, 2592–2598. [Google Scholar] [CrossRef]
- Spinelli, G.; Lamberti, P.; Tucci, V.; Guadagno, L.; Vertuccio, L. Damage Monitoring of Structural Resins Loaded with Carbon Fillers: Experimental and Theoretical Study. Nanomaterials 2020, 10, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vertuccio, L.; De Santis, F.; Pantani, R.; Lafdi, K.; Guadagno, L. Effective de-icing skin using graphene-based flexible heater. Compos. Part B Eng. 2019, 162, 600–610. [Google Scholar] [CrossRef]
- Vertuccio, L.; Guadagno, L.; Spinelli, G.; Lamberti, P.; Tucci, V.; Russo, S. Piezoresistive properties of resin reinforced with carbon nanotubes for health-monitoring of aircraft primary structures. Compos. Part B Eng. 2016, 107, 192–202. [Google Scholar] [CrossRef]
- Vertuccio, L.; Guadagno, L.; Spinelli, G.; Lamberti, P.; Zarrelli, M.; Russo, S.; Iannuzzo, G. Smart coatings of epoxy based CNTs designed to meet practical expectations in aeronautics. Compos. Part B Eng. 2018, 147, 42–46. [Google Scholar] [CrossRef]
- Xian, G.; Karbhari, V.M. Segmental relaxation of water-aged ambient cured epoxy. Polym. Degrad. Stab. 2007, 92, 1650–1659. [Google Scholar] [CrossRef]
- Kusoglu, A.; Kushner, D.; Paul, D.K.; Karan, K.; Hickner, M.; Weber, A.Z. Impact of substrate and processing on confinement of Nafion thin films. Adv. Funct. Mater. 2014, 24, 4763–4774. [Google Scholar] [CrossRef]
- Starkova, O.; Chandrasekaran, S.; Schnoor, T.; Sevcenko, J.; Schulte, K. Anomalous water diffusion in epoxy/carbon nanoparticle composites. Polym. Degrad. Stab. 2019, 164, 127–135. [Google Scholar] [CrossRef]
- Toscano, A.; Pitarresi, G.; Scafidi, M.; Di Filippo, M.; Spadaro, G.; Alessi, S. Water diffusion and swelling stresses in highly crosslinked epoxy matrices. Polym. Degrad. Stab. 2016, 133, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Garden, L.; Pethrick, R.A. A dielectric study of water uptake in epoxy resin systems. J. Appl. Polym. Sci. 2017, 134, 44717. [Google Scholar] [CrossRef]
- Maxwell, I.D.; Pethrick, R.A. Dielectric studies of water in epoxy resins. J. Appl. Polym. Sci. 1983, 28, 2363–2379. [Google Scholar] [CrossRef]
- Ivanova, K.; Pethrick, R.; Affrossman, S. Investigation of hydrothermal ageing of a filled rubber toughened epoxy resin using dynamic mechanical thermal analysis and dielectric spectroscopy. Polymer 2000, 41, 6787–6796. [Google Scholar] [CrossRef]
- Ivanova, K.I.; Pethrick, R.A.; Affrossman, S. Hygrothermal aging of rubber-modified and mineral-filled dicyandiamide-cured DGEBA epoxy resin. II. Dynamic mechanical thermal analysis. J. Appl. Polym. Sci. 2001, 82, 3477–3485. [Google Scholar]
- McConnell, B.K.; Pethrick, A.R. Dielectric studies of water absorption and desorption in epoxy resins: Influence of cure process on behaviour. Polym. Int. 2008, 57, 689–699. [Google Scholar] [CrossRef]
- Guadagno, L.; De Vivo, B.; Di Bartolomeo, A.; Lamberti, P.; Sorrentino, A.; Tucci, V.; Vertuccio, L.; Vittoria, V. Effect of functionalization on the thermo-mechanical and electrical behavior of multi-wall carbon nanotube/epoxy composites. Carbon 2011, 49, 1919–1930. [Google Scholar] [CrossRef]
- Bauhofer, W.; Kovacs, J.Z. A review and analysis of electrical percolation in carbon nanotube polymer composites. Compos. Sci. Technol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Vittoria, V.; Sorrentino, A.; Vertuccio, L.; Raimondo, M.; Tucci, V.; De Vivo, B.; Lamberti, P.; Iannuzzo, G.; et al. Cure behavior and physical properties of epoxy resin—filled with multiwalled carbon nanotubes. J. Nanosci. Nanotechnol. 2010, 10, 2686–2693. [Google Scholar] [CrossRef]
- Kovacs, J.Z.; Velagala, B.S.; Schulte, K.; Bauhofer, W. Two percolation thresholds in carbon nanotube epoxy composites. Compos. Sci. Technol. 2007, 67, 922–928. [Google Scholar] [CrossRef] [Green Version]
- Connor, M.T.; Roy, S.; Ezquerra, T.; Calleja, F.J.B. Broadband ac conductivity of conductor-polymer composites. Phys. Rev. B 1998, 57, 2286–2294. [Google Scholar] [CrossRef] [Green Version]
- Kilbride, B.E.; Coleman, J.; Fraysse, J.; Fournet, P.; Cadek, M.; Drury, A.; Hutzler, S.; Roth, S.; Blau, W. Experimental observation of scaling laws for alternating current and direct current conductivity in polymer-carbon nanotube composite thin films. J. Appl. Phys. 2002, 92, 4024–4030. [Google Scholar] [CrossRef]
- Nan, C.-W.; Shen, Y.; Ma, J. Physical properties of composites near percolation. Annu. Rev. Mater. Res. 2010, 40, 131–151. [Google Scholar] [CrossRef]
- Spinelli, G.; Lamberti, P.; Tucci, V.; Vertuccio, L.; Guadagno, L. Experimental and theoretical study on piezoresistive properties of a structural resin reinforced with carbon nanotubes for strain sensing and damage monitoring. Compos. Part B Eng. 2018, 145, 90–99. [Google Scholar] [CrossRef]
- Vertuccio, L.; Vittoria, V.; Guadagno, L.; De Santis, F. Strain and damage monitoring in carbon-nanotube-based composite under cyclic strain. Compos. Part A Appl. Sci. Manuf. 2015, 71, 9–16. [Google Scholar] [CrossRef]
- Kotsilkova, R.; Fragiadakis, D.; Pissis, P. Reinforcement effect of carbon nanofillers in an epoxy resin system: Rheology, molecular dynamics, and mechanical studies. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 522–533. [Google Scholar] [CrossRef]
- Li, B.; Zhong, W.-H. Review on polymer/graphite nanoplatelet nanocomposites. J. Mater. Sci. 2011, 46, 5595–5614. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Moon, K.-S.; Wong, C.P. Glass transition and relaxation behavior of epoxy nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3849–3858. [Google Scholar] [CrossRef]
- Guadagno, L.; Raimondo, M.; Vittoria, V.; Vertuccio, L.; Naddeo, C.; Russo, S.; De Vivo, B.; Lamberti, P.; Spinelli, G.; Tucci, V. Development of epoxy mixtures for application in aeronautics and aerospace. RSC Adv. 2014, 4, 15474–15488. [Google Scholar] [CrossRef]
- Kim, Y.J.; Shin, T.S.; Choi, H.D.; Kwon, J.H.; Chung, Y.-C.; Yoon, H.G. Electrical conductivity of chemically modified multiwalled carbon nanotube/epoxy composites. Carbon 2005, 43, 23–30. [Google Scholar] [CrossRef]
- Vertuccio, L.; Russo, S.; Raimondo, M.; Lafdi, K.; Guadagno, L. Influence of carbon nanofillers on the curing kinetics of epoxy-amine resin. RSC Adv. 2015, 5, 90437–90450. [Google Scholar] [CrossRef]
- Stimoniaris, A.; Stergiou, C.; Delides, C. A detailed study of α-relaxation in epoxy/carbon nanoparticles composites using computational analysis. Express Polym. Lett. 2012, 6, 120–128. [Google Scholar] [CrossRef]
- Marquardt, D.W. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Maddams, W.F. The scope and limitations of curve fitting. Appl. Spectrosc. 1980, 34, 245–267. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1979. [Google Scholar]
- Li, L.; Yu, Y.; Wu, Q.; Zhan, G.; Li, S. Effect of chemical structure on the water sorption of amine-cured epoxy resins. Corros. Sci. 2009, 51, 3000–3006. [Google Scholar] [CrossRef]
- Wang, J.; Gong, J.; Gong, Z.; Yan, X.; Wang, B.; Wu, Q.; Li, S. Effect of curing agent polarity on water absorption and free volume in epoxy resin studied by PALS. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 2355–2361. [Google Scholar] [CrossRef]
- Maggana, C.; Pissis, P. Water sorption and diffusion studies in an epoxy resin system. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 1165–1182. [Google Scholar] [CrossRef]
- Liu, W.; Hoa, S.; Pugh, M. Water uptake of epoxy–clay nanocomposites: Experiments and model validation. Compos. Sci. Technol. 2008, 68, 2066–2072. [Google Scholar] [CrossRef]
- Bouvet, G.; Cohendoz, S.; Feaugas, X.; Touzain, S.; Mallarino, S. Microstructural reorganization in model epoxy network during cyclic hygrothermal ageing. Polymer 2017, 122, 1–11. [Google Scholar] [CrossRef]
- Kohler, R.; Dück, R.; Ausperger, B.; Alex, R. A numeric model for the kinetics of water vapor sorption on cellulosic reinforcement fibers. Compos. Interfaces 2003, 10, 255–276. [Google Scholar] [CrossRef]
- Kreze, T.; Iskrač, S.; Smole, M.S.; Stana-Kleinschek, K.; Strnad, S.; Fakin, D. Flax fibers sorption properties influenced by different pretreatment processes. J. Nat. Fibers 2005, 2, 25–37. [Google Scholar] [CrossRef]
- Hill, C.A.; Keating, B.A.; Jalaludin, Z.; Mahrdt, E. A rheological description of the water vapour sorption kinetics behaviour of wood invoking a model using a canonical assembly of Kelvin-Voigt elements and a possible link with sorption hysteresis. Holzforschung 2012, 66, 35–47. [Google Scholar] [CrossRef]
- Njuguna, M.; Yan, C.; Hu, N.; Bell, J.; Yarlagadda, P. Sandwiched carbon nanotube film as strain sensor. Compos. Part Ba Eng. 2012, 43, 2711–2717. [Google Scholar] [CrossRef] [Green Version]
- Morris, D.R.P.; Liu, S.P.; Gonzalez, D.V.; Gostick, J.T. Effect of water sorption on the electronic conductivity of porous polymer electrolyte membrane fuel cell catalyst layers. ACS Appl. Mater. Interfaces 2014, 6, 18609–18618. [Google Scholar] [CrossRef] [PubMed]
- Kongkanand, A. Interfacial water transport measurements in Nafion thin films using a quartz-crystal microbalance. J. Phys. Chem. C 2011, 115, 11318–11325. [Google Scholar] [CrossRef]
- Satterfield, M.B.; Benziger, J.B. Non-fickian water vapor sorption dynamics by Nafion membranes. J. Phys. Chem. B 2008, 112, 3693–3704. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.; Ng, Q. Elution behavior of model compounds in gel permeation chromatography. J. Polym. Sci. Polym. Symp. 1968, 21, 105–117. [Google Scholar] [CrossRef]
- Horie, K.; Hiura, H.; Sawada, M.; Mita, I.; Kambe, H. Calorimetric investigation of polymerization reactions. III. Curing reaction of epoxides with amines. J. Polym. Sci. A Polym. Chem. 1970, 8, 1357–1372. [Google Scholar] [CrossRef]
- Karayannidou, E.G.; Achilias, D.S.; Sideridou, I.D. Cure kinetics of epoxy–amine resins used in the restoration of works of art from glass or ceramic. Eur. Polym. J. 2006, 42, 3311–3323. [Google Scholar] [CrossRef]





| Product | Formula | Supplier | Functional Group |
|---|---|---|---|
| DGEBA |  | Sigma Aldrich | 2 |
| DDS |  | Sigma Aldrich | 2 * |
| DMA | Specific |
| Sample dimension | 4 × 10 × 35 mm3 |
| Configuration | Dual Cantilever |
| Displacement amplitude | 0.001 |
| Frequency operating condition | 1 Hz |
| Temperature operating condition | from 30 °C to 300 °C |
| Scanning rate | 3 °C/min−1 |
| Electrical measurement | Specific |
| Sample dimension | 0.5 × 10 × 10 mm3 |
| Configuration | Cabot Test Method (CTM) E043 based on ASTM D4496 |
| Contact (gold) | coating deposition by Agar Auto Sputter Coater |
| Water sorption test | Specific |
| Sample dimension | 0.5 × 10 × 10 mm3 |
| Temperature | 30 °C |
| Environmental condition | Liquid water |
| Image of the Epoxy 0.1CNT sample | |
 | |
| Water Sorption (%) | Tg main peak (°C) | Tg av (°C) | IH (/) | HTAD (%) |
|---|---|---|---|---|
| 0 | 180.1 | 179.2 | 0.95 | 0.00 |
| 1.2 | 171.7 | 169.2 | 0.86 | 9.12 |
| 1.9 | 170.1 | 161.2 | 0.62 | 34.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guadagno, L.; Vertuccio, L. Resistive Response of Carbon Nanotube-Based Composites Subjected to Water Aging. Nanomaterials 2021, 11, 2183. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11092183
Guadagno L, Vertuccio L. Resistive Response of Carbon Nanotube-Based Composites Subjected to Water Aging. Nanomaterials. 2021; 11(9):2183. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11092183
Chicago/Turabian StyleGuadagno, Liberata, and Luigi Vertuccio. 2021. "Resistive Response of Carbon Nanotube-Based Composites Subjected to Water Aging" Nanomaterials 11, no. 9: 2183. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11092183