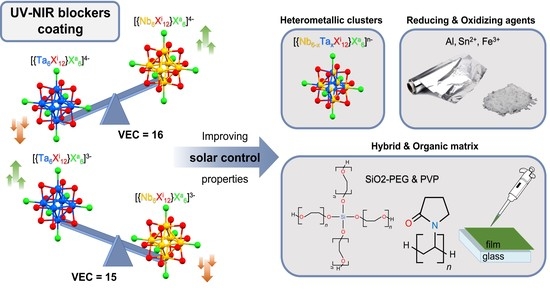
Controlling the Deposition Process of Nanoarchitectonic Nanocomposites Based on {Nb6−xTaxXi12}n+ Octahedral Cluster-Based Building Blocks (Xi = Cl, Br; 0 ≤ x ≤ 6, n = 2, 3, 4) for UV-NIR Blockers Coating Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Study of the Powders and Solutions
3.2. Study of the {Nb6-xTaxXi12}@SiO2-PEG Films
3.3. Study of the {Nb6-xTaxXi12}-Y@PVP Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cotton, F.A. Metal Atom Clusters in Oxide Systems. Inorg. Chem. 1964, 3, 1217–1220. [Google Scholar] [CrossRef]
- Schäfer, H.; Schnering, H.G. Metall-Metall-Bindungen bei niederen Halogeniden, Oxyden und Oxydhalogeniden schwerer Übergangsmetalle Thermochemische und strukturelle Prinzipie. Angew. Chem. 1964, 76, 833–849. [Google Scholar] [CrossRef]
- Fleming, P.B.; Mueller, L.A.; McCarley, R.E. Chemistry of polynuclear metal halides. II. Preparation of polynuclear niobium chloride and bromide. Inorg. Chem. 1967, 6, 1–4. [Google Scholar] [CrossRef]
- Simon, A.; Schnering, H.G.; Schäfer, H. Beiträge zur Chemie der Elemente Niob und Tantal. K4Nb6Cl18 Darstellung, Eigenschaften und Struktur. Z. Anorg. Allg. Chem. 1968, 361, 235–248. [Google Scholar] [CrossRef]
- Parsons, J.A.; Vongvusharintra, A.; Koknat, F.W. High temperature conproportionation of niobium pentahalide and niobium metal; A convenient route to hydrated cluster halides Nb6Cl14·8H2O and Nb6Br14·8H2O. Inorg. Nucl. Chem. Lett. 1972, 8, 281–286. [Google Scholar] [CrossRef]
- Koknat, F.W.; Parsons, J.A.; Vongvusharintra, A. Metal cluster halide complexes. I. Efficient synthesis of hydrated hexanuclear niobium and tantalum cluster halides M6X14.8H2O. Inorg. Chem. 1974, 13, 1699–1702. [Google Scholar] [CrossRef]
- Wilmet, M.; Lebastard, C.; Sciortino, F.; Comby-Zerbino, C.; MacAleese, L.; Chirot, F.; Dugourd, P.; Grasset, F.; Matsushita, Y.; Uchikoshi, T.; et al. Revisiting properties of edge-bridged bromide tantalum clusters in the solid-state, in solution and vice versa: An intertwined experimental and modelling approach. Dalton Trans. 2021, 50, 8002–8016. [Google Scholar] [CrossRef]
- McCarley, R.E.; Hughes, B.G.; Cotton, F.A.; Zimmerman, R. The Two-Electron Oxidation of Metal Atom Cluster Species of the Type [M6X12]2 +. Inorg. Chem. 1965, 4, 1491–1492. [Google Scholar] [CrossRef]
- Espenson, J.H.; McCarley, R.E. Oxidation of Tantalum Cluster Ions. J. Am. Chem. Soc. 1966, 88, 1063–1064. [Google Scholar] [CrossRef]
- Spreckelmeyer, B.; Schäfer, H. Die verbindung Nb6Cl16 3C2H5OH. J. Less Common Met. 1967, 13, 127–129. [Google Scholar] [CrossRef]
- Renaud, A.; Wilmet, M.; Truong, T.G.; Seze, M.; Lemoine, P.; Dumait, N.; Chen, W.; Saito, N.; Ohsawa, T.; Uchikoshi, T.; et al. Transparent tantalum cluster-based UV and IR blocking electrochromic devices. J. Mater. Chem. C 2017, 5, 8160–8168. [Google Scholar] [CrossRef]
- Nguyen, T.K.N.; Renaud, A.; Wilmet, M.; Dumait, N.; Paofai, S.; Dierre, B.; Chen, W.; Ohashi, N.; Cordier, S.; Grasset, F.; et al. New ultra-violet and near-infrared blocking filters for energy saving applications: Fabrication of tantalum metal atom cluster-based nanocomposite thin films by electrophoretic deposition. J. Mater. Chem. C 2017, 5, 10477–10484. [Google Scholar] [CrossRef]
- Chen, W.; Wilmet, M.; Truong, T.G.; Dumait, N.; Cordier, S.; Matsui, Y.; Hara, T.; Takei, T.; Saito, N.; Nguyen, T.K.N.; et al. Embedding hexanuclear tantalum bromide cluster {Ta6Br12} into SiO2 nanoparticles by reverse microemulsion method. Heliyon 2018, 4, e00654. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.N.; Dubernet, M.; Matsui, Y.; Wilmet, M.; Shirahata, N.; Rydzek, G.; Dumait, N.; Amela-Cortes, M.; Renaud, A.; Cordier, S.; et al. Transparent functional nanocomposite films based on octahedral metal clusters: Synthesis by electrophoretic deposition process and characterization. R. Soc. Open Sci. 2019, 6, 181647. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Nguyen, T.K.N.; Wilmet, M.; Dumait, N.; Makrygenni, O.; Matsui, Y.; Takei, T.; Cordier, S.; Ohashi, N.; Uchikoshi, T.; et al. ITO@SiO2 and ITO@{M6Br12}@SiO2 (M = Nb, Ta) nanocomposite films for ultraviolet-near infrared shielding. Nanoscale Adv. 2019, 1, 3693–3698. [Google Scholar] [CrossRef] [Green Version]
- Mullan, B.F.; Madsen, M.T.; Messerle, L.; Kolesnichenko, V.; Kruger, J. X-ray attenuation coefficients of high-atomic-number, hexanuclear transition metal cluster compounds: A new paradigm for radiographic contrast agents. Acad. Radiol. 2000, 7, 254–259. [Google Scholar] [CrossRef]
- Löwe, J.; Stock, D.; Jap, B.; Zwickl, P.; Baumeister, W.; Huber, R. Crystal Structure of the 20S Proteasome from the Archaeon, T. acidophilum at 3.4 Å. Resolution. Science 1995, 268, 533–539. [Google Scholar] [CrossRef]
- Cramer, P.; Bushnell, D.A.; Fu, J.; Gnatt, A.L.; Maier-Davis, B.; Thompson, N.E.; Burgess, R.R.; Edwards, A.M.; David, P.R.; Kornberg, R.D. Architecture of RNA Polymerase II and Implications for the Transcription Mechanism. Science 2000, 288, 640–649. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, K.N.; Iverson, T.M.; Maghlaoui, K.; Barber, J.; Iwata, S. Architecture of the Photosynthetic Oxygen-Evolving Center. Science 2004, 303, 1831–1838. [Google Scholar] [CrossRef] [Green Version]
- Preetz, W.; Harder, K. Trennung und Charakterisierung der metallgemischten Cluster [(NbnTa6–n)Cli12]2 +, n = 0–6. Z. Anorg. Allg. Chem. 1991, 597, 163–172. [Google Scholar] [CrossRef]
- Wilmet, M. Elaboration de Revêtements Transparents à Base de Clusters de Métaux de Transition pour le Blocage des Rayonnements Proche-Infrarouge. Ph.D. Thesis, Rennes 1 University, Rennes, France, 2018. Available online: http://www.theses.fr/2018REN1S108 (accessed on 24 October 2018).
- Lebastard, C. Synthèses, Caractérisations et Mise en Forme de Matériaux à Clusters Pour la Conception de Vitrage à Contrôle Solaire. Ph.D. Thesis, Rennes 1 University, Rennes, France, 2021. Available online: http://www.theses.fr/s211547 (accessed on 3 November 2021).
- Prokopowicz, M. Silica-Polyethylene Glycol Matrix Synthesis by Sol-Gel Method and Evaluation for Diclofenac Diethyloammonium Release. Drug Deliv. 2007, 14, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Feng, L.; Wang, C.; Zhao, W.; Li, X. Confinement effect of SiO2 framework on phase change of PEG in shape-stabilized PEG/SiO2 composites. Eur. Polym. J. 2012, 48, 803–810. [Google Scholar] [CrossRef]
- Catauro, M.; Mozzati, M.C.; Bollino, F. Sol–gel hybrid materials for aerospace applications: Chemical characterization and comparative investigation of the magnetic properties. Acta Astronaut. 2015, 117, 153–162. [Google Scholar] [CrossRef]
- Chabrié, P.C. Sur un nouveau chlorure de tantale. Compt. Rend. 1907, 144, 804. [Google Scholar]
- Van Haagen, W.K. Tantalum and Some of Its Halides. Ph.D. Thesis, University of Pennsylvania, Philadelphia, PA, USA, 1909. [Google Scholar]
- Chapin, W.H. Halide bases of tantalum. J. Am. Chem. Soc. 1910, 32, 323–330. [Google Scholar] [CrossRef]
- Eisenbraun, R.; Schäfer, H. Drei Wege zur Oxydation von [Ta6Cl12]2+ ([Ta6Br12]2+ und [Nb6Cl12]2+). Z. Anorg. Allg. Chem. 1985, 530, 222–226. [Google Scholar] [CrossRef]
- Lebastard, C.; Wilmet, W.; Cordier, S.; Comby-Zerbino, C.; MacAleese, L.; Dugourd, P.; Uchikoshi, T.; Dorcet, V.; Amela-Cortes, M.; Renaud, A.; et al. Nanoarchitectonics of glass coatings for near-infrared shielding: From solid-state cluster-based niobium chlorides to the shaping of nanocomposite films. ACS Appl. Mater. Interfaces 2022, 14, 21116–21130. [Google Scholar] [CrossRef]
- Lebastard, C.; Wilmet, M.; Cordier, S.; Comby-Zerbino, C.; MacAleese, L.; Dugourd, P.; Ohashi, N.; Uchikoshi, T.; Grasset, F. Optimized coating of ITO@glass by {Nb5TaX12}@PVP nanocomposites (X = Cl, Br) for solar glazing applications. Sci. Technol. Adv. Mater. 2022. submitted. [Google Scholar]
- Li, D.; Liu, Z.; Wang, Y.; Shan, Y.; Huang, F. Efficiency enhancement of Cu(In,Ga)Se2 solar cells by applying SiO2–PEG/PVP antireflection coatings. J. Mater. Sci. Technol. 2015, 31, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Li, H.; Klimov, V.I. Tandem luminescent solar concentrators based on engineered quantum dots. Nat. Photon. 2018, 12, 105–110. [Google Scholar] [CrossRef]
- Sanchez, C.; Lebeau, B.; Chaput, F.; Boilot, J.P. Optical Properties of Functional Hybrid Organic–Inorganic Nanocomposites. Adv. Mater. 2003, 15, 1969–1994. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Gallicchio, M.; Pacifico, S. Influence of the polymer amount on bioactivity and biocompatibility of SiO2/PEG hybrid materials synthesized by sol-gel technique. Mater. Sci. Eng. C 2015, 48, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.W.S.; Cook, K.D. Mass spectrometric study of interactions between poly (ethylene glycols) and alkali metals in solution. Macromolecules 1983, 16, 1736–1740. [Google Scholar] [CrossRef]
- Verma, S.; Jain, S.L. Complexation of KBr3 with poly(ethylene glycol): Efficient bromination of aromatics under solvent-less conditions. Organic. Chem. Current. Res. 2012, 1, 1000105. [Google Scholar] [CrossRef] [Green Version]
- Mroczka, R.; Słodkowska, A. The properties of the polyethylene glycol complex PEG(Na+)(Cu+) on the copper electrodeposited layer by time-of-flight secondary-ion mass spectrometry. The new insights. Electrochim. Acta 2020, 339, 135931. [Google Scholar] [CrossRef]
- Aubert, T.; Nerambourg, N.; Saito, N.; Haneda, H.; Ohashi, N.; Mortier, M.; Cordier, S.; Grasset, F. Tunable Visible Emission of Luminescent Hybrid Nanoparticles Incorporating Two Complementary Luminophores: ZnO Nanocrystals and [Mo6Br14]2− Nanosized Cluster Units. Part. Part. Syst. Charact. 2013, 30, 90–95. [Google Scholar] [CrossRef]
- Aslan, M.; Weingarth, D.; Jäckel, N.; Atchison, J.S.; Grobelsek, I.; Presser, V. Polyvinylpyrrolidone as binder for castable supercapacitor electrodes with high electrochemical performance in organic electrolytes. J. Power Sources 2014, 266, 374–383. [Google Scholar] [CrossRef]
- Selvam, S.; Balamuralitharan, B.; Karthick, S.N.; Dennyson Savariraj, A.; Hemalatha, K.V.; Kim, S.K.; Kim, H.J. Novel high-temperature supercapacitor combined dye sensitized solar cell from sulfated β-cyclodextrin/PVP/MnCO3 composite. J. Mater. Chem. A 2015, 3, 10225–10232. [Google Scholar] [CrossRef]
- Truong, T.G.; Dierre, B.; Grasset, F.; Saito, N.; Saito, N.; Nguyen, T.K.N.; Takahashi, K.; Uchikoshi, T.; Amela-Cortes, M.; Molard, Y.; et al. Visible tunable lighting system based on polymer composites embedding ZnO and metallic clusters: From colloids to thin films. Sci. Technol. Adv. Mater. 2016, 17, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Zha, H.; Liu, G.; Han, G. High-performance laminated luminescent solar concentrators based on colloidal carbon quantum dots. Nanoscale Adv. 2019, 1, 4888–4894. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, X.; Zhang, Y.; Dong, R.; Luscombe, C.K. Review on the Role of Polymers in Luminescent Solar Concentrators. J. Polym. Sci. A Polym. Chem. 2019, 57, 201–215. [Google Scholar] [CrossRef] [Green Version]
- Kotok, V.; Vadym, K.A. Study of the Influence of Polyvinyl Pyrrolidone Concentration in the Deposition Electrolyte on the Properties of Electrochromic Ni(OH)2 Films. East. Eur. J. Enterp. Technol. 2020, 4, 31–37. [Google Scholar] [CrossRef]
- Saeed, A.M.N.; Hezam, A.; Al-Gunaid, M.Q.A.; Somesh, T.E.; Siddaramaiah, B. Effect of ethylene carbonate on properties of PVP-CsAlO2-LiClO4 solid polymer electrolytes. Polym. Plast. Technol. Mater. 2021, 60, 132–146. [Google Scholar] [CrossRef]
- Kim, A.; Hosseinmardi, A.; Annamalai, P.K.; Kumar, P.; Patel, R. Review on Colloidal Quantum Dots Luminescent Solar Concentrators. ChemistrySelect 2021, 6, 4948–4967. [Google Scholar] [CrossRef]
- Lin, G.; Luo, F.; Pan, H.; Smedskjaer, M.M.; Teng, Y.; Chen, D.; Qiu, J.; Zhao, Q. Universal Preparation of Novel Metal and Semiconductor Nanoparticle–Glass Composites with Excellent Nonlinear Optical Properties. J. Phys. Chem. C 2011, 115, 24598–24604. [Google Scholar] [CrossRef]
- Smith, G.B.; Deller, C.A.; Swift, P.D.; Gentle, A.; Garrett, P.D.; Fisher, W.K. Nanoparticle-doped Polymer Foils for Use in Solar Control Glazing. J. Nanoparticle Res. 2002, 4, 157–165. [Google Scholar] [CrossRef]
- Schelm, S.; Smith, G.B.; Garrett, P.D.; Fisher, W.K. Tuning the surface-plasmon resonance in nanoparticles for glazing applications. J. Appl. Phys. 2005, 97, 124314. [Google Scholar] [CrossRef]
- Stokes, N.L.; Edgar, J.A.; McDonagh, A.M.; Cortie, M.B. Spectrally selective coatings of gold nanorods on architectural glass. J. Nanopart. Res. 2010, 12, 2821–2830. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Wu, X.; Huang, T. Greatly improved NIR shielding performance of CuS nanocrystals by gallium doping for energy efficient window. Ceram. Int. 2021, 47, 23827–23833. [Google Scholar] [CrossRef]
- Ariga, K.; Nishikawa, M.; Mori, T.; Takeya, J.; Kumar Shrestha, L.; Hill, J.P. Self-assembly as a key player for materials nanoarchitectonics. Sci. Technol. Adv. Mater. 2019, 20, 51–95. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Gibbons, T.H.; Cortie, M.B. Spectrally-selective Gold Nanorod Coatings for Window Glass. Gold Bull. 2006, 39, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Wang, S.; Luo, H.; Dai, L.; Cao, C.; Liu, Y.; Chen, Z.; Kanehira, M. Enhanced chemical stability of VO2 nanoparticles by the formation of SiO2/VO2 core/shell structures and the application to transparent and flexible VO2-based composite foils with excellent thermochromic properties for solar heat control. Energy Environ. Sci. 2012, 5, 6104–6110. [Google Scholar] [CrossRef]
- Garcia, G.; Buonsanti, R.; Llordes, A.; Runnerstrom, E.L.; Bergerud, A.; Milliron, D.J. Near-Infrared Spectrally Selective Plasmonic Electrochromic Thin Films. Adv. Opt. Mater. 2013, 1, 215–220. [Google Scholar] [CrossRef]
- Kang, L.; Xu, W.; Wang, K.; Liang, W.; Liu, X.; Gao, F.; Lan, A.; Yang, Y.; Gao, Y. Transparent (NH4)xWO3 colloidal dispersion and solar control foils: Low temperature synthesis, oxygen deficiency regulation and NIR shielding ability. Sol. Energy Mater. Sol. Cells 2014, 128, 184–189. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Liang, J.; Yu, X.; Li, D. Tunable Solar-Heat Shielding Property of Transparent Films Based on Mesoporous Sb-Doped SnO2 Microspheres. ACS Appl. Mater. Interfaces 2015, 7, 6574–6583. [Google Scholar] [CrossRef] [PubMed]
- Carboni, M.; Carravetta, M.; Zhang, X.L.; Stulz, E. Efficient NIR light blockage with matrix embedded silver nanoprism thin films for energy saving window coating. J. Mater. Chem. C 2016, 4, 1584–1588. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.T.K.; Renaud, A.; Dierre, B.; Bouteille, B.; Wilmet, M.; Dubernet, M.; Ohashi, N.; Grasset, F.; Uchikoshi, T. Extended Study on Electrophoretic Deposition Process of Inorganic Octahedral Metal Clusters: Advanced Multifunctional Transparent Nanocomposite Thin Films. Bull. Chem. Soc. Jpn. 2018, 91, 1763–1774. [Google Scholar] [CrossRef] [Green Version]
- Stiles, W.S.; Burch, J.M. NPL colour-matching investigation: Final report (1958). Opt. Acta Int. J. Opt. 1959, 6, 1–26. [Google Scholar] [CrossRef]
- Speranskaya, N.I. Determination of spectral color co-ordinates for twenty-seven normal observers. Opt. Spectroscopy 1959, 7, 424–428. Available online: https://0-www-scopus-com.brum.beds.ac.uk/record/display.uri?eid=2-s2.0-0001307974&origin=inward&txGid=160a89521936d5c60bb09d3e4dc95d35 (accessed on 4 May 2022).
- Judd, D.B. Proc. CIE Symposium on “Advanced Colorimetry”; CIE Central Bureau: Vienna, Austria, 1993; p. 107. Available online: https://cie.co.at/publications/proceedings-cie-symposium-advanced-colorimetry-8-10-june-1993-vienna-austria (accessed on 4 May 2022).
- Trezona, P.W. Derivation of the 1964 CIE 10° XYZ colour-matching functions and their applicability in photometry. Color Res. Appl. 2001, 26, 67–75. [Google Scholar] [CrossRef]
- Lindbloom, B.J. Site Gathered the Useful Color Information. 2017. Available online: http://www.Brucelindbloom.com (accessed on 4 May 2022).












| Compositions of the Cluster Core in PVP | TL | TE | TL/TE | x | y | z | SNIR (%) |
|---|---|---|---|---|---|---|---|
| {Nb6Cli12}-12(VEC = 16) [30] | 62.2 | 48.3 | 1.29 | 0.38 | 0.43 | 0.19 | 53.7 |
| {Nb6Cli12}-10(VEC = 15) | 57.1 | 58.2 | 0.98 | 0.42 | 0.47 | 0.11 | 32.0 |
| {Nb5TaCli12}-16(VEC = 16) [31] | 54.1 | 40.7 | 1.33 | 0.37 | 0.43 | 0.20 | 54.4 |
| {Nb4Ta2Cli12}-16(VEC = 16) | 58.1 | 46.2 | 1.26 | 0.34 | 0.41 | 0.26 | 46.2 |
| {Nb3Ta3Cli12}-16(VEC = 16) | 61.5 | 50.8 | 1.21 | 0.33 | 0.39 | 0.27 | 40.2 |
| {Nb2Ta4Cli12}-12(VEC = 16) | 60.6 | 57.1 | 1.06 | 0.28 | 0.36 | 0.36 | 29.3 |
| {NbTa5Cli12}-12(VEC = 16) | 52.6 | 52.7 | 1.00 | 0.26 | 0.36 | 0.38 | 28.6 |
| {Ta6Cli12}-10(VEC = 16) | 55.6 | 52.7 | 1.05 | 0.27 | 0.37 | 0.36 | 30.7 |
| {Nb6Bri12}-12(VEC = 16) | 48.8 | 37.6 | 1.29 | 0.45 | 0.49 | 0.10 | 58.6 |
| {Nb5TaBri12}-12(VEC = 16) [31] | 52.7 | 40.4 | 1.30 | 0.38 | 0.46 | 0.17 | 47.6 |
| {Nb4Ta2Bri12}-8(VEC = 16) | 58.6 | 47.5 | 1.23 | 0.34 | 0.42 | 0.24 | 33.1 |
| {Nb3Ta3Bri12}-12(VEC = 16) | 53.9 | 45.6 | 1.18 | 0.34 | 0.43 | 0.24 | 40.8 |
| {Nb2Ta4Bri12}-8(VEC = 16) | 55.0 | 49.8 | 1.10 | 0.31 | 0.41 | 0.29 | 33.0 |
| {NbTa5Bri12}-12(VEC = 16) | 50.2 | 45.5 | 1.10 | 0.32 | 0.42 | 0.26 | 35.2 |
| {Ta6Bri12}-12(VEC = 16) | 55.7 | 58.1 | 0.95 | 0.28 | 0.42 | 0.30 | 18.4 |
| {Ta6Bri12}-10(VEC = 15) | 71.2 | 56.1 | 1.27 | 0.37 | 0.42 | 0.21 | 35.8 |
| @PVP on glass | 91.4 | 90.5 | 1.01 | 0.31 | 0.33 | 0.35 | 8.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebastard, C.; Wilmet, M.; Cordier, S.; Comby-Zerbino, C.; MacAleese, L.; Dugourd, P.; Hara, T.; Ohashi, N.; Uchikoshi, T.; Grasset, F. Controlling the Deposition Process of Nanoarchitectonic Nanocomposites Based on {Nb6−xTaxXi12}n+ Octahedral Cluster-Based Building Blocks (Xi = Cl, Br; 0 ≤ x ≤ 6, n = 2, 3, 4) for UV-NIR Blockers Coating Applications. Nanomaterials 2022, 12, 2052. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12122052
Lebastard C, Wilmet M, Cordier S, Comby-Zerbino C, MacAleese L, Dugourd P, Hara T, Ohashi N, Uchikoshi T, Grasset F. Controlling the Deposition Process of Nanoarchitectonic Nanocomposites Based on {Nb6−xTaxXi12}n+ Octahedral Cluster-Based Building Blocks (Xi = Cl, Br; 0 ≤ x ≤ 6, n = 2, 3, 4) for UV-NIR Blockers Coating Applications. Nanomaterials. 2022; 12(12):2052. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12122052
Chicago/Turabian StyleLebastard, Clément, Maxence Wilmet, Stéphane Cordier, Clothilde Comby-Zerbino, Luke MacAleese, Philippe Dugourd, Toru Hara, Naoki Ohashi, Tetsuo Uchikoshi, and Fabien Grasset. 2022. "Controlling the Deposition Process of Nanoarchitectonic Nanocomposites Based on {Nb6−xTaxXi12}n+ Octahedral Cluster-Based Building Blocks (Xi = Cl, Br; 0 ≤ x ≤ 6, n = 2, 3, 4) for UV-NIR Blockers Coating Applications" Nanomaterials 12, no. 12: 2052. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12122052