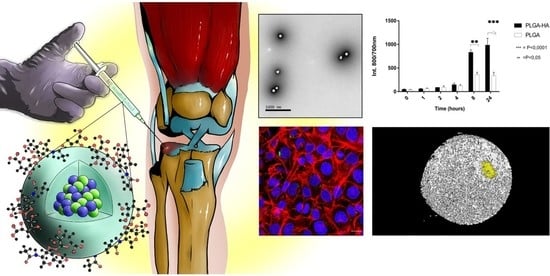
PLGA Nanoparticles Grafted with Hyaluronic Acid to Improve Site-Specificity and Drug Dose Delivery in Osteoarthritis Nanotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Synthesis
2.2.1. Synthesis and Characterization of PLGA-Grafted Sodium Hyaluronate Copolymer
2.2.2. Chemical Characterization of the Synthesized Polymer
2.2.3. Quantification of Hyaluronic Acid
2.3. Preparation of Gold Nanoparticles
2.4. Preparation of PLGA and PLGA-HA NPs
2.5. Characterization of Nanoparticles
2.5.1. Encapsulation Efficiency Analysis of NIR Dye
2.5.2. Encapsulation Efficiency Analysis of FITC Dye
2.5.3. Encapsulation Efficiency Analysis of Gold
2.5.4. Particle Size and Charge Surface
2.5.5. Particle Surface and Morphology
2.6. Release Studies of NIR Dye and Gold from NPs
2.6.1. Release Study of NIR Dye and Gold in PBS
2.6.2. Release Studies of NIR Dye and Gold in Synovial Fluid
2.7. In Vitro Characterization of Nanoparticles
2.7.1. Cell Culture
2.7.2. Cell Metabolic Assay (MTS)
2.7.3. Cell Binding Analysis by Odyssey
2.8. NPs Uptake Studies
2.8.1. Uptake Characterized by Odyssey
2.8.2. Uptake Characterized by Flow Cytometry
2.8.3. Uptake Characterized by Confocal Microscopy
2.9. In Vivo Characterization of NPs
2.9.1. Animals and Experimental Design
2.9.2. NPs Retention Time in the Mouse Knee Joint
2.9.3. Detection of NPs in Mouse Knee Joint by CT Scans
2.10. Ex Vivo Characterization of NPs
NPs Targeting Studies on Human Cartilage Explants
2.11. Statistical Analysis
3. Results and Discussions
3.1. Synthesis and Characterization of PLGA-Grafted Hyaluronate Copolymers
3.2. NPs Formulation and Characterizations
Release Studies
3.3. Characterization of the NPs in the In Vitro Experiments
3.3.1. Cellular Metabolic Assay and Chondrocytes Binding Assay
3.3.2. NPs’ Uptake by Chondrocytes
3.3.3. NPs’ Retention in Mice Joint (In Vivo Study)
3.3.4. The Target Specificity of NPs in Human Cartilage Explants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Ouyang, H.; Dass, C.R.; Xu, J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016, 4, 15040. [Google Scholar] [CrossRef]
- Freitas-Silva, R.; Conde, D.M.; Freitas-Júnior, R.d.; Martinez, E.Z. Comparison of quality of life, satisfaction with surgery and shoulder-arm morbidity in breast cancer survivors submitted to breast-conserving therapy or mastectomy followed by immediate breast reconstruction. Clinics 2010, 65, 781–787. [Google Scholar] [CrossRef] [Green Version]
- Święchowicz, S.; Ostałowska, A.; Kasperczyk, A.; Nowak, D.; Birkner, E.; Kasperczyk, S. Evaluation of hyaluronic acid intra-articular injections in the treatment of primary and secondary osteoarthritis of the knee. Med. Sci. Monit. 2012, 77, 105–109. [Google Scholar] [PubMed]
- Holyoak, D.T.; Tian, Y.F.; van der Meulen, M.C.H.; Singh, A. Osteoarthritis: Pathology, Mouse Models, and Nanoparticle Injectable Systems for Targeted Treatment. Ann. Biomed. Eng. 2016, 44, 2062–2075. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.L.; Im, G.-I. Drug delivery systems for intra-articular treatment of osteoarthritis. Expert Opin. Drug Deliv. 2014, 11, 269–282. [Google Scholar] [CrossRef]
- Altman, R.D.; Manjoo, A.; Fierlinger, A.; Niazi, F.; Nicholls, M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: A systematic review. BMC Musculoskelet. Disord. 2015, 16, 321. [Google Scholar] [CrossRef] [Green Version]
- Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236. [Google Scholar] [CrossRef]
- Laroui, H.; Grossin, L.; Léonard, M.; Stoltz, J.-F.; Gillet, P.; Netter, P.; Dellacherie, E. Hyaluronate-Covered Nanoparticles for the Therapeutic Targeting of Cartilage. Biomacromolecules 2007, 8, 3879–3885. [Google Scholar] [CrossRef]
- Zille, H.; Paquet, J.; Henrionnet, C.; Scala-Bertola, J.; Leonard, M.; Six, J.L.; Deschamp, F.; Netter, P.; Vergès, J.; Gillet, P.; et al. Evaluation of intra-articular delivery of hyaluronic acid functionalized biopolymeric nanoparticles in healthy rat knees. Bio-Med. Mater. Eng. 2010, 20, 235–242. [Google Scholar] [CrossRef]
- Jeong, Y.-I.; Kim, D.H.; Chung, C.-W.; Yoo, J.J.; Choi, K.H.; Kim, C.H.; Ha, S.H.; Kang, D.H. Self-assembled nanoparticles of hyaluronic acid/poly(dl-lactide-co-glycolide) block copolymer. Colloids Surf. B Biointerfaces 2012, 90, 28–35. [Google Scholar] [CrossRef]
- Hilderbrand, S.A.; Weissleder, R. Near-infrared fluorescence: Application to in vivo molecular imaging. Curr. Opin. Chem. Biol. 2010, 14, 71–79. [Google Scholar] [CrossRef]
- Xi, D.; Dong, S.; Meng, X.; Lu, Q.; Meng, L.; Ye, J. Gold nanoparticles as computerized tomography (CT) contrast agents. RSC Adv. 2012, 2, 12515–12524. [Google Scholar] [CrossRef]
- Hornos Carneiro, M.F.; Barbosa, F. Gold nanoparticles: A critical review of therapeutic applications and toxicological aspects. J. Toxicol. Environ. Health Part B 2016, 19, 129–148. [Google Scholar] [CrossRef]
- Chen, M.; Daddy, J.C.K.A.; Xiao, Y.; Ping, Q.; Zong, L. Advanced nanomedicine for rheumatoid arthritis treatment: Focus on active targeting. Expert Opin. Drug Deliv. 2017, 14, 1141–1144. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Ahn, C.-H.; Park, T.G. Poly[lactic-co-(glycolic acid)]-Grafted Hyaluronic Acid Copolymer Micelle Nanoparticles for Target-Specific Delivery of Doxorubicin. Macromol. Biosci. 2009, 9, 336–342. [Google Scholar] [CrossRef]
- Yoo, H.S.; Park, T.G. Folate receptor targeted biodegradable polymeric doxorubicin micelles. J. Control. Release 2004, 96, 273–283. [Google Scholar] [CrossRef]
- Oueslati, N.; Leblanc, P.; Harscoat-Schiavo, C.; Rondags, E.; Meunier, S.; Kapel, R.; Marc, I. CTAB turbidimetric method for assaying hyaluronic acid in complex environments and under cross-linked form. Carbohydr. Polym. 2014, 112, 102–108. [Google Scholar] [CrossRef]
- Song, J.-M.; Im, J.-H.; Kang, J.-H.; Kang, D.-J. A simple method for hyaluronic acid quantification in culture broth. Carbohydr. Polym. 2009, 78, 633–634. [Google Scholar] [CrossRef]
- Zerrillo, L.; Que, I.; Vepris, O.; Morgado, L.N.; Chan, A.; Bierau, K.; Li, Y.; Galli, F.; Bos, E.; Censi, R.; et al. pH-responsive poly(lactide-co-glycolide) nanoparticles containing near-infrared dye for visualization and hyaluronic acid for treatment of osteoarthritis. J. Control. Release 2019, 309, 265–276. [Google Scholar] [CrossRef]
- Claassen, H.; Schicht, M.; Brandt, J.; Reuse, K.; Schädlich, R.; Goldring, M.B.; Guddat, S.S.; Thate, A.; Paulsen, F. C-28/I2 and T/C-28a2 chondrocytes as well as human primary articular chondrocytes express sex hormone and insulin receptors—Useful cells in study of cartilage metabolism. Ann. Anat.-Anat. Anz. 2011, 193, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Goldring, M.B. Culture of Immortalized Chondrocytes and Their Use As Models of Chondrocyte Function. In Cartilage and Osteoarthritis: Volume 1: Cellular and Molecular Tools; Sabatini, M., Pastoureau, P., De Ceuninck, F., Eds.; Humana Press: Totowa, NJ, USA, 2004; pp. 37–52. [Google Scholar]
- Torchilin, V.P. Passive and Active Drug Targeting: Drug Delivery to Tumors as an Example. In Drug Delivery; Schäfer-Korting, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–53. [Google Scholar]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vangara, K.K.; Liu, J.L.; Palakurthi, S. Hyaluronic Acid-decorated PLGA-PEG Nanoparticles for Targeted Delivery of SN-38 to Ovarian Cancer. Anticancer Res. 2013, 33, 2425–2434. [Google Scholar] [PubMed]
- Chen, E.Y.; Liu, W.F.; Megido, L.; Díez, P.; Fuentes, M.; Fager, C.; Olsson, E.; Gessner, I.; Mathur, S. Chapter 3—Understanding and utilizing the biomolecule/nanosystems interface. In Nanotechnologies in Preventive and Regenerative Medicine; Uskoković, V., Uskoković, D.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 207–297. [Google Scholar]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knudson, W.; Loeser, R.F. CD44 and integrin matrix receptors participate in cartilage homeostasis. Cell. Mol. Life Sci. CMLS 2002, 59, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Ishida, O.; Tanaka, Y.; Morimoto, I.; Takigawa, M.; Eto, S. Chondrocytes Are Regulated by Cellular Adhesion Through CD44 and Hyaluronic Acid Pathway. J. Bone Miner. Res. 1997, 12, 1657–1663. [Google Scholar] [CrossRef]
- Saneja, A.; Nayak, D.; Srinivas, M.; Kumar, A.; Khare, V.; Katoch, A.; Goswami, A.; Vishwakarma, R.A.; Sawant, S.D.; Gupta, P.N. Development and mechanistic insight into enhanced cytotoxic potential of hyaluronic acid conjugated nanoparticles in CD44 overexpressing cancer cells. Eur. J. Pharm. Sci. 2017, 97, 79–91. [Google Scholar] [CrossRef]








| NPs | Particle Size (nm) | PDI | ζ-Potential (mV) | EE% (NIR) | EE% (Gold) | EE% (FITC) |
|---|---|---|---|---|---|---|
| PLGA | 228 ± 1 | 0.31 ± 0.05 | −15 ± 2 | 84.3 | 16.1 | 24.6 |
| PLGA-HA | 200 ± 2 | 0.6 ± 0.2 | −23 ± 2 | 65.3 | 8.4 | 10.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerrillo, L.; Gigliobianco, M.R.; D’Atri, D.; Garcia, J.P.; Baldazzi, F.; Ridwan, Y.; Fuentes, G.; Chan, A.; Creemers, L.B.; Censi, R.; et al. PLGA Nanoparticles Grafted with Hyaluronic Acid to Improve Site-Specificity and Drug Dose Delivery in Osteoarthritis Nanotherapy. Nanomaterials 2022, 12, 2248. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12132248
Zerrillo L, Gigliobianco MR, D’Atri D, Garcia JP, Baldazzi F, Ridwan Y, Fuentes G, Chan A, Creemers LB, Censi R, et al. PLGA Nanoparticles Grafted with Hyaluronic Acid to Improve Site-Specificity and Drug Dose Delivery in Osteoarthritis Nanotherapy. Nanomaterials. 2022; 12(13):2248. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12132248
Chicago/Turabian StyleZerrillo, Luana, Maria Rosa Gigliobianco, Domenico D’Atri, Joao Pedro Garcia, Fabio Baldazzi, Yanto Ridwan, Gastón Fuentes, Alan Chan, Laura B. Creemers, Roberta Censi, and et al. 2022. "PLGA Nanoparticles Grafted with Hyaluronic Acid to Improve Site-Specificity and Drug Dose Delivery in Osteoarthritis Nanotherapy" Nanomaterials 12, no. 13: 2248. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12132248