Designed Mini Protein 20 Mimicking Uricase Encapsulated in ZIF-8 as Nanozyme Biosensor for Uric Acid Detection
Abstract
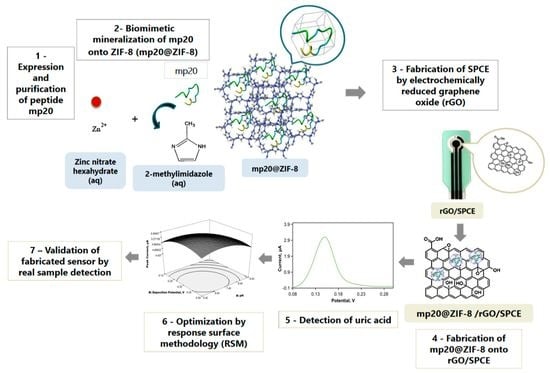
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instruments
2.3. Methodology
2.3.1. Synthesis of mp20@ZIF-8 Biocomposite
2.3.2. Functionalization of mp20@ZIF-8/rGO/SPCE Electrode
2.3.3. Experimental Design on the Optimization Condition of Uric Acid Detection Based on Response Surface Methodology (RSM)
2.3.4. Verification of the Model
2.3.5. Electrochemical Detection of Uric Acid by Differential Pulse Voltammetry (DPV) Analysis
3. Results and Discussion
3.1. Powder X-ray Diffraction (PXRD) Analysis
3.2. Surface Analysis by Raman Spectroscopy and FESEM
3.3. Electrochemical Properties of Modified Electrodes
3.4. Statistical Analysis of the Results and Model Fitting
3.5. Effect of Each Factor
3.6. Effect of Interaction between Factors
3.7. Validation of the Predictive Model and Optimization of the Current Response
3.8. Comparative Study and Analytical Performance of mp20@ZIF-8/rGO/SPCE Electrode towards Uric Acid
3.9. Selectivity, Reproducibility, and Stability Studies
3.10. Real Physiological Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dey, N.; Bhattacharya, S. Nanomolar Level Detection of Uric Acid in Blood Serum and Pest-Infested Grain Samples by an Amphiphilic Probe. Anal. Chem. 2017, 89, 10376–10383. [Google Scholar] [CrossRef] [PubMed]
- Ponnaiah, S.K.; Periakaruppan, P.; Vellaichamy, B. New Electrochemical Sensor Based on a Silver-Doped Iron Oxide Nanocomposite Coupled with Polyaniline and Its Sensing Application for Picomolar-Level Detection of Uric Acid in Human Blood and Urine Samples. J. Phys. Chem. B 2018, 122, 3037–3046. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Zhi, N.; Yang, L.; Xu, G.; Feng, Q.; Zhang, Q.; Sun, S. A highly sensitive uric acid electrochemical biosensor based on a nano-cube cuprous oxide/ferrocene/uricase modified glassy carbon electrode. Sci. Rep. 2020, 10, 10607. [Google Scholar] [CrossRef]
- Su, C.-H.; Sun, C.-L.; Liao, Y.-C. Printed Combinatorial Sensors for Simultaneous Detection of Ascorbic Acid, Uric Acid, Dopamine, and Nitrite. ACS Omega 2017, 2, 4245–4252. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, R.; Tripathy, N.; Ahn, M.S.; Hahn, Y.B. Solution Process Synthesis of High Aspect Ratio ZnO Nanorods on Electrode Surface for Sensitive Electrochemical Detection of Uric Acid. Sci. Rep. 2017, 7, 46475. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Qi, X.; Zhang, G.; Wang, S.; Li, K.; Wu, J.; Wan, X.; Liu, Y.; Li, Q. Low-cost voltammetric sensors for robust determination of toxic Cd (II) and Pb (II) in environment and food based on shuttle-like α-Fe2O3 nanoparticles decorated β-Bi2O3 microspheres. Microchem. J. 2022, 179, 107515. [Google Scholar] [CrossRef]
- Li, G.; Qi, X.; Wu, J.; Xu, L.; Wan, X.; Liu, Y.; Chen, Y.; Li, Q. Ultrasensitive, label-free voltammetric determination of norfloxacin based on molecularly imprinted polymers and Au nanoparticle-functionalized black phosphorus nanosheet nanocomposite. J. Hazard. Mater. 2022, 436, 129107. [Google Scholar] [CrossRef]
- Wang, C.; Sudlow, G.; Wang, Z.; Cao, S.; Jiang, Q.; Neiner, A.; Morrissey, J.J.; Kharasch, E.D.; Achilefu, S.; Singamaneni, S. Metal-Organic Framework Encapsulation Preserves the Bioactivity of Protein Therapeutics. Adv. Healthc. Mater. 2018, 7, 1800950. [Google Scholar] [CrossRef]
- Wang, C.; Sun, H.; Luan, J.; Jiang, Q.; Tadepalli, S.; Morrissey, J.J.; Kharasch, E.D.; Singamaneni, S. Metal–Organic Framework Encapsulation for Biospecimen Preservation. Chem. Mater. 2018, 30, 1291–1300. [Google Scholar] [CrossRef]
- Srivastava, A.; Singh, V.; Haque, S.; Pandey, S.; Mishra, M.; Jawed, A.; Shukla, P.K.; Singh, P.K.; Tripathi, C.K.M. Response Surface Methodology-Genetic Algorithm Based Medium Optimization, Purification, and Characterization of Cholesterol Oxidase from Streptomyces rimosus. Sci. Rep. 2018, 8, 10913. [Google Scholar] [CrossRef] [Green Version]
- Kaith, B.S.; Sharma, R.; Kalia, S.; Bhatti, M.S. Response surface methodology and optimized synthesis of guar gum-based hydrogels with enhanced swelling capacity. RSC Adv. 2014, 4, 40339–40344. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, H.; Liu, G.; Wang, Z. Box–Behnken response surface design for the optimization of electrochemical detection of cadmium by Square Wave Anodic Stripping Voltammetry on bismuth film/glassy carbon electrode. Sens. Actuators B Chem. 2016, 235, 67–73. [Google Scholar] [CrossRef]
- Wu, X.; Yang, C.; Ge, J. Green synthesis of enzyme/metal-organic framework composites with high stability in protein denaturing solvents. Bioresour. Bioprocess. 2017, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ge, J.; Yang, C.; Hou, M.; Liu, Z. Facile synthesis of multiple enzyme-containing metal–organic frameworks in a biomolecule-friendly environment. Chem. Commun. 2015, 51, 13408–13411. [Google Scholar] [CrossRef]
- Liang, K.; Ricco, R.; Doherty, C.M.; Styles, M.J.; Bell, S.; Kirby, N.; Mudie, S.; Haylock, D.; Hill, A.J.; Doonan, C.J.; et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 2015, 6, 7240. [Google Scholar] [CrossRef] [Green Version]
- Samadi-Maybodi, A.; Ghasemi, S.; Ghaffari-Rad, H. A novel sensor based on Ag-loaded zeolitic imidazolate framework-8 nanocrystals for efficient electrocatalytic oxidation and trace level detection of hydrazine. Sens. Actuators B Chem. 2015, 220, 627–633. [Google Scholar] [CrossRef]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef]
- Raj, M.A.; John, S.A. Simultaneous determination of uric acid, xanthine, hypoxanthine and caffeine in human blood serum and urine samples using electrochemically reduced graphene oxide modified electrode. Anal. Chim. Acta 2013, 771, 14–20. [Google Scholar] [CrossRef]
- Palanisamy, S.; Chen, S.-M.; Sarawathi, R. A novel nonenzymatic hydrogen peroxide sensor based on reduced graphene oxide/ZnO composite modified electrode. Sens. Actuators B Chem. 2012, 166, 372–377. [Google Scholar] [CrossRef]
- Zainal, N.; Ahmad, S.; Ngee, L. Surface Modification of Screen-Printed Carbon Electrode (SPCE) with Calixarene-Functionalized Electrochemically Reduced Graphene Oxide (ERGO/C4) in the Electrochemical Detection of Anthracene. J. Electrochem. Soc. 2019, 166, B110–B116. [Google Scholar] [CrossRef]
- Liang, K.; Carbonell, C.; Styles, M.J.; Ricco, R.; Cui, J.; Richardson, J.J.; Maspoch, D.; Caruso, F.; Falcaro, P. Biomimetic replication of microscopic metal–organic framework patterns using printed protein patterns. Adv. Mater. 2015, 27, 7293–7298. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Gao, C.; Chen, L.; He, Y.; Ma, W.; Lin, Z. Recent advances in biomolecule immobilization based on self-assembly: Organic–inorganic hybrid nanoflowers and metal–organic frameworks as novel substrates. J. Mater. Chem. B 2018, 6, 1581–1594. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.; Banerjee, S.; Lollar, C.; Wang, X.; Zhou, H.-C. Enzyme–MOF (metal–organic framework) composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Eksin, E.; Congur, G. Indicator-free electrochemical biosensor for microRNA detection based on carbon nanofibers modified screen printed electrodes. J. Electroanal. Chem. 2015, 755, 167–173. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Moreira, F.T.; Fernandes, R.; Sales, M.G.F. Novel and simple electrochemical biosensor monitoring attomolar levels of miRNA-155 in breast cancer. Biosens. Bioelectron. 2016, 80, 621–630. [Google Scholar] [CrossRef]
- Pan, D.; Gu, Y.; Lan, H.; Sun, Y.; Gao, H. Functional graphene-gold nano-composite fabricated electrochemical biosensor for direct and rapid detection of bisphenol A. Anal. Chim. Acta 2015, 853, 297–302. [Google Scholar] [CrossRef]
- Sulaiman, I.S.C.; Basri, M.; Masoumi, H.R.F.; Ashari, S.E.; Ismail, M. Design and development of a nanoemulsion system containing extract of Clinacanthus nutans (L.) leaves for transdermal delivery system by D-optimal mixture design and evaluation of its physicochemical properties. RSC Adv. 2016, 6, 67378–67388. [Google Scholar] [CrossRef]
- Khairudin, N.; Basri, M.; Fard Masoumi, H.R.; Samson, S.; Ashari, S.E. Enhancing the bioconversion of azelaic acid to its derivatives by response surface methodology. Molecules 2018, 23, 397. [Google Scholar] [CrossRef] [Green Version]
- Gunawan, E.R.; Basri, M.; Rahman, M.B.A.; Salleh, A.B.; Rahman, R.N.Z. A Study on response surface methodology (RSM) of lipase-catalyzed synthesis of palm-based wax ester. Enzym. Microb. Technol. 2005, 37, 739–744. [Google Scholar] [CrossRef]
- Song, C.; Li, X.; Wang, L.; Shi, W. Fabrication, characterization and response surface method (RSM) optimization for tetracycline photodegration by Bi 3.84 W 0.16 O 6.24-graphene oxide (BWO-GO). Sci. Rep. 2016, 6, 37466. [Google Scholar] [CrossRef] [Green Version]
- Babaei, A.; Zendehdel, M.; Khalilzadeh, B.; Taheri, A. Simultaneous determination of tryptophan, uric acid and ascorbic acid at iron (III) doped zeolite modified carbon paste electrode. Colloids Surf. B Biointerfaces 2008, 66, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Vinoth, V.; Wu, J.J.; Anandan, S. Sensitive electrochemical determination of dopamine and uric acid using AuNPs (EDAS)–rGO nanocomposites. Anal. Methods 2016, 8, 4379–4390. [Google Scholar] [CrossRef]
- Abd Rahim, A.S.M. Design of Mini Protein that Mimics Uricase in the Preliminary Development of Nanowire-Based Uric Acid Biosensor. Ph.D. Thesis, Universiti Putra Malaysia, Serdang, Malaysia, 2017. [Google Scholar]
- Tukimin, N.; Abdullah, J.; Sulaiman, Y. Electrodeposition of poly (3, 4-ethylenedioxythiophene)/reduced graphene oxide/manganese dioxide for simultaneous detection of uric acid, dopamine and ascorbic acid. J. Electroanal. Chem. 2018, 820, 74–81. [Google Scholar] [CrossRef]
- Crosnier de Lassichere, C.; Latapie, L.; Evrard, D.; Gros, P. New Insight into the EC’Mechanism of Uric Acid Regeneration in the Presence of Ascorbic Acid on a Poly (3, 4-ethylenedioxithiophene) Modified Gold Electrode. Electroanalysis 2018, 30, 1653–1658. [Google Scholar] [CrossRef]
- Miland, E.; Ordieres, A.M.; Blanco, P.T.; Smyth, M.R.; Fagain, C.O. Poly (o-aminophenol)-modified bienzyme carbon paste electrode for the detection of uric acid. Talanta 1996, 43, 785–796. [Google Scholar] [CrossRef]
- Çete, S.; Yaşar, A.; Arslan, F. An amperometric biosensor for uric acid determination prepared from uricase immobilized in polypyrrole film. Artif. Cells Blood Substit. Biotechnol. 2006, 34, 367–380. [Google Scholar] [CrossRef]
- Omar, M.N.; Salleh, A.B.; Lim, H.N.; Tajudin, A.A. Electrochemical detection of uric acid via uricase-immobilized graphene oxide. Anal. Biochem. 2016, 509, 135–141. [Google Scholar] [CrossRef]
- Rawal, R.; Chawla, S.; Chauhan, N.; Dahiya, T.; Pundir, C.S. Construction of amperometric uric acid biosensor based on uricase immobilized on PBNPs/cMWCNT/PANI/Au composite. Int. J. Biol. Macromol. 2012, 50, 112–118. [Google Scholar] [CrossRef]
- Jain, S.; Verma, S.; Singh, S.P.; Sharma, S.N. An electrochemical biosensor based on novel butylamine capped CZTS nanoparticles immobilized by uricase for uric acid detection. Biosens. Bioelectron. 2019, 127, 135–141. [Google Scholar] [CrossRef]
- Atta, N.F.; El-Kady, M.F.; Galal, A. Simultaneous determination of catecholamines, uric acid and ascorbic acid at physiological levels using poly (N-methylpyrrole)/Pd-nanoclusters sensor. Anal. Biochem. 2010, 400, 78–88. [Google Scholar] [CrossRef]
- Dmytruk, K.V.; Smutok, O.V.; Dmytruk, O.V.; Schuhmann, W.; Sibirny, A.A. Construction of uricase-overproducing strains of Hansenula polymorpha and its application as biological recognition element in microbial urate biosensor. BMC Biotechnol. 2011, 11, 58. [Google Scholar] [CrossRef] [PubMed]









| Variables | Value |
|---|---|
| Standard Deviation (SD) | 0.038 |
| Mean | 4.57 |
| Coefficient of Variation (CV) | 0.84 |
| Predicted Residual Error Sum of Squares (PRESS) | 0.14 |
| R2 | 0.9992 |
| Adjusted R2 | 0.9983 |
| Predicted R2 | 0.9904 |
| Adequate Precision | 85.389 |
| Number | pH | Deposition Potential, V | Deposition Time, s | Current, μA | RSE (%) | |
|---|---|---|---|---|---|---|
| Predicted | Experiment | |||||
| 1 | 7.4 | −0.35 | 56.56 | 5.20 | 5.19 | 0.19 |
| 2 | 7.4 | −0.3 | 50.56 | 5.09 | 5.00 | 1.76 |
| 3 | 7.4 | −0.4 | 46.56 | 5.10 | 5.08 | 0.39 |
| 4 | 7.4 | −0.2 | 66.56 | 4.88 | 4.90 | 0.41 |
| 5 | 7.4 | −0.45 | 40.56 | 4.85 | 4.86 | 0.21 |
| Fabricated Sensor | Linear Range (μM) | Detection Limit (μM) | Ref. |
|---|---|---|---|
| UOX-HRP, entrapment, carbon paste covered with poly(o-aminophenol) | Up to 100 | 3.0 | [36] |
| UOX, glutaraldehyte cross-linking, polypyrrole membrane | 1.0–50 | 0.5 | [37] |
| uricase onto GO | 20–490 | 3.45 | [38] |
| immobilizing uricase in crosslinked chitosan network through glutaraldehyde, onto PBNPs/c-MWCNT/PANI/Au modified electrode | 5–800 | 5.0 | [39] |
| UOx/EDC:NHS/CZTS/ITO- bioelectrode | 50–700 | 0.066 | [40] |
| Uricase-overproducing strains of Hansenula polymorpha on graphite electrode | Up to 180 | 8.0 | [41] |
| mp20 encapsulated ZIF-8 on reduced graphene oxide (mp20@ZIF-8/rGO/SPCE) electrode | 1–34 | 0.27 | present work |
| Signal Changed (%) | RSD (%) | |
|---|---|---|
| Interferent | ||
| Ascorbic Acid | 1.73 | 0.83 |
| Urea | 3.56 | 0.75 |
| Glucose | 3.08 | 2.68 |
| L-Cysteine | 2.70 | 3.46 |
| Creatinine | 0.71 | 1.64 |
| Stability (Days) | ||
| 14 | 4 | 1.0 |
| 30 | 7 | 2.5 |
| 60 | 7 | 0.1 |
| 180 | 8 | 1.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Aziz, S.F.N.; Salleh, A.B.; Ashari, S.E.; Normi, Y.M.; Yusof, N.A.; Alang Ahmad, S.A. Designed Mini Protein 20 Mimicking Uricase Encapsulated in ZIF-8 as Nanozyme Biosensor for Uric Acid Detection. Nanomaterials 2022, 12, 2290. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12132290
Abdul Aziz SFN, Salleh AB, Ashari SE, Normi YM, Yusof NA, Alang Ahmad SA. Designed Mini Protein 20 Mimicking Uricase Encapsulated in ZIF-8 as Nanozyme Biosensor for Uric Acid Detection. Nanomaterials. 2022; 12(13):2290. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12132290
Chicago/Turabian StyleAbdul Aziz, Siti Fatimah Nur, Abu Bakar Salleh, Siti Efliza Ashari, Yahaya M. Normi, Nor Azah Yusof, and Shahrul Ainliah Alang Ahmad. 2022. "Designed Mini Protein 20 Mimicking Uricase Encapsulated in ZIF-8 as Nanozyme Biosensor for Uric Acid Detection" Nanomaterials 12, no. 13: 2290. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12132290