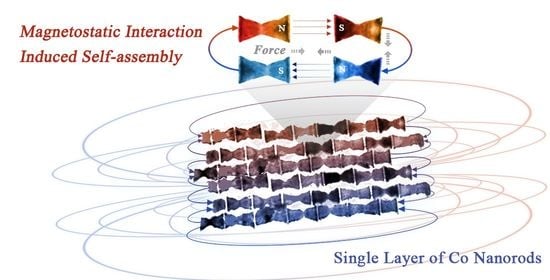
A Self-Assembly of Single Layer of Co Nanorods to Reveal the Magnetostatic Interaction Mechanism
Abstract
:1. Introduction
2. Experiments and Methods
2.1. Chemicals and Materials
2.2. The Synthesis of Cobalt (II) Laurate
2.3. The Synthesis of Cobalt Nanorods Using a Solvothermal Route
2.4. The Synthesis of Cobalt Nanorods Using an Alcohol–Thermal Method
2.5. The Self-Assembly of Co Nanorods
2.6. Characterization
3. Results and Discussions
3.1. The Self-Assembly of Co Nanorods with Cambered Tips
3.2. The Self-Assembly of Co Nanorods with Flat Tips
3.3. The Discussion of Magnetostatic Interaction Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, Z.H.; Yue, M.; Liu, H.; Yin, Z.Y.; Wei, K.C.; Guan, H.Q.; Lin, H.H.; Shen, M.Q.; An, S.Z.; Wu, Q.; et al. Stabilizing hard magnetic SmCo5 nanoparticles by n-doped graphitic carbon layer. J. Am. Chem. Soc. 2020, 142, 8440–8446. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, J.; Li, W.; Sun, S.; Gao, S.; Hou, Y. Magnetic nanostructures: Rational design and fabrication strategies toward diverse applications. Chem. Rev. 2022, 122, 5411–5475. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Luo, Y.; Yu, D.B.; Peng, H.J.; Yan, W.L.; Wang, Z.L.; Bai, X.Y. Preparation and properties of hot-deformed magnets processed from nanocrystalline/amorphous Nd-Fe-B powders. Rare Met. 2021, 40, 2033–2039. [Google Scholar] [CrossRef]
- Ma, Z.; Tian, H.; Cong, L.; Wu, Q.; Yue, M.; Sun, S. A flame-reaction method for the large-scale synthesis of high-performance SmxCoy nanomagnets. Angew. Chem.-Int. Ed. 2019, 58, 14509–14512. [Google Scholar] [CrossRef]
- Yang, W.; Liang, D.; Kong, X.; Yang, J. Neutron diffraction studies of permanent magnetic materials. Rare Met. 2020, 39, 13–21. [Google Scholar] [CrossRef]
- Shen, B.; Yu, C.; Baker, A.A.; McCall, S.K.; Yu, Y.; Su, D.; Yin, Z.; Liu, H.; Li, J.; Sun, S. Chemical synthesis of magnetically hard and strong rare earth metal based nanomagnets. Angew. Chem.-Int. Ed. 2019, 58, 602–606. [Google Scholar] [CrossRef]
- Ma, Z.; Du, H. Stabilizing interface of SmCo5/Co nanocomposites by graphene shells. Rare Met. 2022, 41, 1223–1229. [Google Scholar] [CrossRef]
- Xu, G.; Lu, H.; Guo, K.; Tang, F.; Song, X. Predictions on the phase constitution of SmCo7-XMx alloys by data mining. Nanomaterials 2022, 12, 1452. [Google Scholar] [CrossRef]
- Guan, H.Q.; Huang, S.S.; Ding, J.H.; Tian, F.Y.; Xu, Q.; Zhao, J.J. Chemical environment and magnetic moment effects on point defect formations in CoCrNi-based concentrated solid-solution alloys. Acta Mater. 2020, 187, 122–134. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Z.; Sun, M.; Yu, Y.; Wang, J.; Cai, S.B. The influence of the temperature on the dynamic behaviors of magnetorheological gel. Adv. Eng. Mater. 2020, 2101680. [Google Scholar] [CrossRef]
- Lu, C.J.; Zhou, H.; Li, L.F.; Yang, A.C.; Xu, C.B.; Ou, Z.Y.; Wang, J.Q.; Wang, X.; Tian, F. Split-core magnetoelectric current sensor and wireless current measurement application. Measurement 2022, 188, 110527. [Google Scholar] [CrossRef]
- Li, S.M.; Zhang, S.Q.; Zhao, R.Q. Regulating the electronic and magnetic properties of 1T’-ReS2 by fabricating nanoribbons and transition-metal doping: A theoretical study. Nanoscale 2022, 14, 8454–8462. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Kramer, M.; Zhou, L.; Liu, F.; Gabay, A.; Hadjipanayis, G.; Balasubramanian, B.; Sellmyer, D. Current progress and future challenges in rare-earth-free permanent magnets. Acta Mater. 2018, 158, 118–137. [Google Scholar] [CrossRef]
- Li, H.; Wu, Q.; Yue, M.; Peng, Y.; Li, Y.; Liang, J.; Wang, D.; Zhang, J. Magnetization reversal in cobalt nanowires with combined magneto-crystalline and shape anisotropies. J. Magn. Magn. Mater. 2019, 481, 104–110. [Google Scholar] [CrossRef]
- Jia, Y.; Wu, Y.; Zhao, S.; Zuo, S.; Skokov, K.P.; Gutfleisch, O.; Jiang, C.; Xu, H. L1(0) rare-earth-free permanent magnets: The effects of twinning versus dislocations in Mn-Al magnets. Phys. Rev. Mater. 2020, 4, 094402. [Google Scholar] [CrossRef]
- Crisan, A.D.; Leca, A.; Bartha, C.; Dan, I.; Crisan, O. Magnetism and epsilon-tau phase transformation in MnAl-based nanocomposite magnets. Nanomaterials 2021, 11, 896. [Google Scholar] [CrossRef]
- Ma, Z.; Mohapatra, J.; Wei, K.; Liu, J.P.; Sun, S. Magnetic nanoparticles: Synthesis, anisotropy, and applications. Chem. Rev. 2021. [Google Scholar] [CrossRef]
- Proenca, M.P. Multifunctional magnetic nanowires and nanotubes. Nanomaterials 2022, 12, 1308. [Google Scholar] [CrossRef]
- Ma, Z.; Yue, M.; Wu, Q.; Li, C.; Yu, Y. Designing shape anisotropic SmCo5 particles by chemical synthesis to reveal the morphological evolution mechanism. Nanoscale 2018, 10, 10377–10382. [Google Scholar] [CrossRef]
- Mohapatra, J.; Xing, M.; Elkins, J.; Liu, J.P. Hard and semi-hard magnetic materials based on cobalt and cobalt alloys. J. Alloys Compd. 2020, 824, 153874. [Google Scholar] [CrossRef]
- Ma, Z.; Liang, J.; Ma, W.; Cong, L.; Wu, Q.; Yue, M. Chemically synthesized anisotropic SmCo5 nanomagnets with a large energy product. Nanoscale 2019, 11, 12484–12488. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, T.; Jiang, C. A facile synthesis of high performance SmCo5 nanoparticles. Chem. Eng. J. 2015, 264, 610–616. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, R.; Yue, M.; Liu, W. Microstructure and magnetic properties of SmCo5 sintered magnets. Rare Met. 2020, 39, 1295–1299. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, D.; Yoo, D.; Park, S.; Cha, H.; Kwon, H.; Lee, J.; Lee, D. Enhancement of magnetic properties of hot pressed/die-upset Dy-free Nd-Fe-B magnets with Cu/Nd coating by wet process. Rare Met. 2020, 39, 48–54. [Google Scholar] [CrossRef]
- Zeng, H.; Zheng, M.; Skomski, R.; Sellmyer, D.J.; Liu, Y.; Menon, L.; Bandyopadhyay, S. Magnetic properties of self-assembled Co nanowires of varying length and diameter. J. Appl. Phys. 2000, 87, 4718–4720. [Google Scholar] [CrossRef] [Green Version]
- Gandha, K.; Mohapatra, J.; Liu, J.P. Coherent magnetization reversal and high magnetic coercivity in Co nanowire assemblies. J. Magn. Magn. Mater. 2017, 438, 41–45. [Google Scholar] [CrossRef]
- Gandha, K.; Elkins, K.; Poudyal, N.; Liu, X.; Liu, J.P. High energy product developed from cobalt nanowires. Sci. Rep. 2014, 4, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Anagnostopoulou, E.; Grindi, B.; Lacroix, L.M.; Ott, F.; Panagiotopoulos, I.; Viau, G. Dense arrays of cobalt nanorods as rare-earth free permanent magnets. Nanoscale 2016, 8, 4020–4029. [Google Scholar] [CrossRef]
- Ener, S.; Anagnostopoulou, E.; Dirba, I.; Lacroix, L.-M.; Ott, F.; Blon, T.; Piquemal, J.-Y.; Skokov, K.P.; Gutfleisch, O.; Viau, G. Consolidation of cobalt nanorods: A new route for rare-earth free nanostructured permanent magnets. Acta Mater. 2018, 145, 290–297. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Wu, Q.; Yue, M.; Xu, H.; Palaka, S.; Elkins, K.; Liu, J.P. Manipulation of morphology and magnetic properties in cobalt nanowires. AIP Adv. 2017, 7, 056229. [Google Scholar] [CrossRef]
- Patil, S.; Kate, P.R.; Deshpande, J.B.; Kulkarni, A.A. Quantitative understanding of nucleation and growth kinetics of silver nanowires. Chem. Eng. J. 2021, 414, 128711. [Google Scholar] [CrossRef]
- Amirjani, A.; Fatmehsari, D.H.; Marashi, P. Interactive effect of agitation rate and oxidative etching on growth mechanisms of silver nanowires during polyol process. J. Exp. Nanosci. 2015, 10, 1387–1400. [Google Scholar] [CrossRef]
- Li, H.; Wu, Q.; Peng, Y.; Xu, H.; Zhang, J.; Yue, M. Magnetic properties and magnetization reversal in Co nanowires with different morphology. J. Magn. Magn. Mater. 2019, 469, 203–210. [Google Scholar] [CrossRef]
- Mrad, K.; Schoenstein, F.; Nong, H.T.T.; Anagnostopoulou, E.; Viola, A.; Mouton, L.; Mercone, S.; Ricolleau, C.; Jouini, N.; Abderraba, M.; et al. Control of the crystal habit and magnetic properties of Co nanoparticles through the stirring rate. Crystengcomm 2017, 19, 3476–3484. [Google Scholar] [CrossRef]
- Jamet, M.; Wernsdorfer, W.; Thirion, C.; Mailly, D.; Dupuis, V.; Melinon, P.; Perez, A. Magnetic anisotropy of a single cobalt nanocluster. Phys. Rev. Lett. 2001, 86, 4676–4679. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Liu, H.; Yue, M. Magnetically recyclable Sm2Co17/Cu catalyst to chemoselectively reduce the 3-nitrostyrene into 3-vinylaniline under room temperature. Nano Res. 2019, 12, 3085–3088. [Google Scholar] [CrossRef]
- Wu, Q.; Cong, L.; Yue, M.; Li, C.; Ma, Z.; Ma, X.; Wang, Y. A unique synthesis of rare-earth-Co-based single crystal particles by “self-aligned” Co nano-arrays. Nanoscale 2020, 12, 13958–13963. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Zhang, M.; Yang, K.; Li, B.; Ma, Z. A Self-Assembly of Single Layer of Co Nanorods to Reveal the Magnetostatic Interaction Mechanism. Nanomaterials 2022, 12, 2499. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12142499
Du H, Zhang M, Yang K, Li B, Ma Z. A Self-Assembly of Single Layer of Co Nanorods to Reveal the Magnetostatic Interaction Mechanism. Nanomaterials. 2022; 12(14):2499. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12142499
Chicago/Turabian StyleDu, Hongyu, Min Zhang, Ke Yang, Baohe Li, and Zhenhui Ma. 2022. "A Self-Assembly of Single Layer of Co Nanorods to Reveal the Magnetostatic Interaction Mechanism" Nanomaterials 12, no. 14: 2499. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12142499