Bioethanol Conversion into Propylene over Various Zeolite Catalysts: Reaction Optimization and Catalyst Deactivation
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of the Different Zeolite Catalysts
2.2. Characterization
2.3. Ethanol Conersion Reaction
3. Results
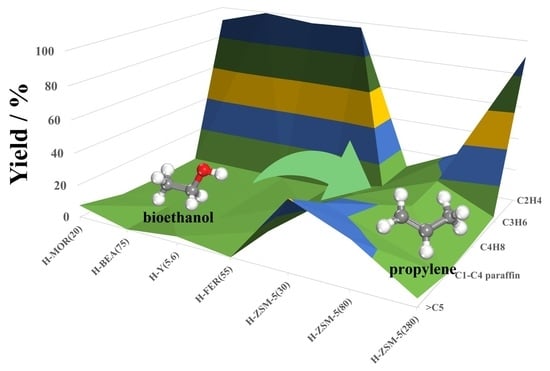
3.1. Ethanol Conversion over different Zeolite Catalysts
3.2. Conversion of Ethanol over H-ZSM-5(80) Catalysts
3.2.1. Calcination Temperature
3.2.2. Feed Composition
3.2.3. Reaction Temperature
3.2.4. Time on Stream
3.2.5. Contact Time
3.2.6. Raw Material
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Morschbacker, A. Bio-Ethanol Based Ethylene. Polym. Rev. 2009, 49, 79–84. [Google Scholar] [CrossRef]
- Phung, T.K.; Pham, T.L.M.; Vu, K.B.; Busca, G. (Bio)Propylene production processes: A critical review. J. Environ. Chem. Eng. 2021, 9, 105673. [Google Scholar] [CrossRef]
- Song, Z.; Liu, W.; Chen, C.; Takahashi, A.; Fujitani, T. Production of propylene from ethanol over ZSM-5 co-modified with zirconium and phosphorus. React. Kinet. Mech. Catal. 2013, 109, 221–231. [Google Scholar] [CrossRef]
- Xia, W.; Chen, K.; Takahashi, A.; Li, X.; Mu, X.; Han, C.; Liu, L.; Nakamura, I.; Fujitani, T. Effects of particle size on catalytic conversion of ethanol to propylene over H-ZSM-5 catalysts—Smaller is better. Catal. Commun. 2016, 73, 27–33. [Google Scholar] [CrossRef]
- Xia, W.; Wang, F.; Mu, X.; Chen, K.; Takahashi, A.; Nakamura, I.; Fujitani, T. Catalytic performance of H-ZSM-5 zeolites for conversion of ethanol or ethylene to propylene: Effect of reaction pressure and SiO2/Al2O3 ratio. Catal. Commun. 2017, 91, 62–66. [Google Scholar] [CrossRef]
- Xia, W.; Wang, J.; Wang, L.; Qian, C.; Ma, C.; Huang, Y.; Fan, Y.; Hou, M.; Chen, K. Ethylene and propylene production from ethanol over Sr/ZSM-5 catalysts: A combined experimental and computational study. Appl. Catal. B Environ. 2021, 294, 120242. [Google Scholar] [CrossRef]
- Inoue, K.; Okabe, K.; Inaba, M.; Takahara, I.; Murata, K. Metal modification effects on ethanol conversion to propylene by H-ZSM-5 with Si/Al2 ratio of 150. React. Kinet. Mech. Catal. 2010, 101, 477–489. [Google Scholar] [CrossRef]
- Schulz, J.; Bandermann, F. Conversion of ethanol over zeolite H-ZSM-5. Chem. Eng. Technol. 1994, 17, 179–186. [Google Scholar] [CrossRef]
- Aguayo, A.T.; Gayubo, A.G.; Tarrío, A.M.; Atutxa, A.; Bilbao, J. Study of operating variables in the transformation of aqueous ethanol into hydrocarbons on an HZSM-5 zeolite. J. Chem. Technol. Biotechnol. 2002, 77, 211–216. [Google Scholar] [CrossRef]
- Inaba, M.; Murata, K.; Saito, M.; Takahara, I. Production of olefins from ethanol by Fe-supported zeolite catalysts. Green Chem. 2007, 9, 638–646. [Google Scholar] [CrossRef]
- Fernandes Machado, N.R.C.; Calsavara, V.; Astrath, N.G.C.; Neto, A.M.; Baesso, M.L. Hydrocarbons from ethanol using [Fe,Al]ZSM-5 zeolites obtained by direct synthesis. Appl. Catal. A Gen. 2006, 311, 193–198. [Google Scholar] [CrossRef]
- Calsavara, V.; Baesso, M.L.; Fernandes-Machado, N.R.C. Transformation of ethanol into hydrocarbons on ZSM-5 zeolites modified with iron in different ways. Fuel 2008, 87, 1628–1636. [Google Scholar] [CrossRef]
- Murata, K.; Inaba, M.; Takahara, I. Effects of Surface Modification of H-ZSM-5 Catalysts on Direct Transformation of Ethanol into Lower Olefins. J. Jpn. Pet. Inst. 2008, 51, 234–239. [Google Scholar] [CrossRef] [Green Version]
- Johansson, R.; Hruby, S.L.; Rass-Hansen, J.; Christensen, C.H. The Hydrocarbon Pool in Ethanol-to-Gasoline over HZSM-5 Catalysts. Catal. Lett. 2008, 127, 1. [Google Scholar] [CrossRef]
- Oikawa, H.; Shibata, Y.; Inazu, K.; Iwase, Y.; Murai, K.; Hyodo, S.; Kobayashi, G.; Baba, T. Highly selective conversion of ethene to propene over SAPO-34 as a solid acid catalyst. Appl. Catal. A Gen. 2006, 312, 181–185. [Google Scholar] [CrossRef]
- Iwamoto, M.; Kosugi, Y.J.J.O.P.C.C. Highly Selective Conversion of Ethene to Propene and Butenes on Nickel Ion-Loaded Mesoporous Silica Catalysts. J. Phys. Chem. C 2007, 111, 13–15. [Google Scholar] [CrossRef]
- Jin, H.; Miao, C.; Yue, Y.; Tian, C.; Hua, W.; Gao, Z. Sm-CeO2/Zeolite Bifunctional Catalyst for Direct and Highly Selective Conversion of Bioethanol to Propylene. Catalysts 2022, 12, 407. [Google Scholar] [CrossRef]
- Jin, H.; Yue, Y.; Miao, C.; Tian, C.; Hua, W.; Gao, Z. Direct and Highly Selective Conversion of Bioethanol to Propylene Over Y-CeO2 and Zeolite Beta Composite. Catal. Lett. 2022. [Google Scholar] [CrossRef]
- Jin, H.; Xu, D.; Tian, C.; Yue, Y.; Hua, W.; Gao, Z. Insights into Promoting Effect of Sm on Catalytic Performance of the CeO2/Beta Catalyst in Direct Conversion of Bioethanol to Propylene. Chem. Res. Chin. Univ. 2022. [Google Scholar] [CrossRef]
- Inoue, T.; Itakura, M.; Jon, H.; Oumi, Y.; Takahashi, A.; Fujitani, T.; Sano, T. Synthesis of LEV zeolite by interzeolite conversion method and its catalytic performance in ethanol to olefins reaction. Microporous Mesoporous Mater. 2009, 122, 149–154. [Google Scholar] [CrossRef]
- Makarfi, Y.I.; Yakimova, M.S.; Lermontov, A.S.; Erofeev, V.I.; Koval, L.M.; Tretiyakov, V.F. Conversion of bioethanol over zeolites. Chem. Eng. J. 2009, 154, 396–400. [Google Scholar] [CrossRef]
- Inaba, M.; Murata, K.; Takahara, I. Effect of Fe-loading and reaction temperature on the production of olefins from ethanol by Fe/H-ZSM-5 zeolite catalysts. React. Kinet. Catal. Lett. 2009, 97, 19–26. [Google Scholar] [CrossRef]
- Takahashi, A.; Xia, W.; Nakamura, I.; Shimada, H.; Fujitani, T. Effects of added phosphorus on conversion of ethanol to propylene over ZSM-5 catalysts. Appl. Catal. A Gen. 2012, 423–424, 162–167. [Google Scholar] [CrossRef]
- Song, Z.; Takahashi, A.; Mimura, N.; Fujitani, T. Production of Propylene from Ethanol Over ZSM-5 Zeolites. Catal. Lett. 2009, 131, 364–369. [Google Scholar] [CrossRef]
- Song, Z.; Takahashi, A.; Nakamura, I.; Fujitani, T. Phosphorus-modified ZSM-5 for conversion of ethanol to propylene. Appl. Catal. A Gen. 2010, 384, 201–205. [Google Scholar] [CrossRef]
- Müller, J.M.; Mesquita, G.C.; Franco, S.M.; Borges, L.D.; de Macedo, J.L.; Dias, J.A.; Dias, S.C.L. Solid-state dealumination of zeolites for use as catalysts in alcohol dehydration. Microporous Mesoporous Mater. 2015, 204, 50–57. [Google Scholar] [CrossRef]
- Van der Borght, K.; Galvita, V.V.; Marin, G.B. Ethanol to higher hydrocarbons over Ni, Ga, Fe-modified ZSM-5: Effect of metal content. Appl. Catal. A Gen. 2015, 492, 117–126. [Google Scholar] [CrossRef]
- Phung, T.K.; Radikapratama, R.; Garbarino, G.; Lagazzo, A.; Riani, P.; Busca, G. Tuning of product selectivity in the conversion of ethanol to hydrocarbons over H-ZSM-5 based zeolite catalysts. Fuel Processing Technol. 2015, 137, 290–297. [Google Scholar] [CrossRef]
- Phung, T.K.; Proietti Hernández, L.; Lagazzo, A.; Busca, G. Dehydration of ethanol over zeolites, silica alumina and alumina: Lewis acidity, Brønsted acidity and confinement effects. Appl. Catal. A Gen. 2015, 493, 77–89. [Google Scholar] [CrossRef]
- Phung, T.K.; Busca, G. Diethyl ether cracking and ethanol dehydration: Acid catalysis and reaction paths. Chem. Eng. J. 2015, 272, 92–101. [Google Scholar] [CrossRef]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, W.; Ma, C.; Huang, Y.; Li, S.; Wang, X.; Chen, K.; Liu, D. Bioethanol Conversion into Propylene over Various Zeolite Catalysts: Reaction Optimization and Catalyst Deactivation. Nanomaterials 2022, 12, 2746. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12162746
Xia W, Ma C, Huang Y, Li S, Wang X, Chen K, Liu D. Bioethanol Conversion into Propylene over Various Zeolite Catalysts: Reaction Optimization and Catalyst Deactivation. Nanomaterials. 2022; 12(16):2746. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12162746
Chicago/Turabian StyleXia, Wei, Chao Ma, Yaxin Huang, Shuangshuang Li, Xue Wang, Kun Chen, and Dong Liu. 2022. "Bioethanol Conversion into Propylene over Various Zeolite Catalysts: Reaction Optimization and Catalyst Deactivation" Nanomaterials 12, no. 16: 2746. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12162746