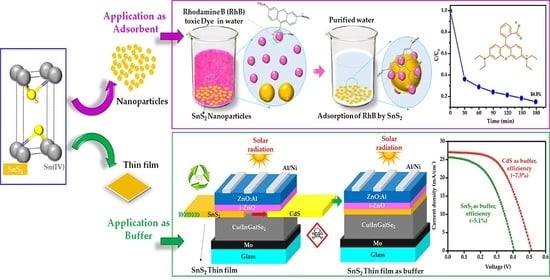
SnS2 Nanoparticles and Thin Film for Application as an Adsorbent and Photovoltaic Buffer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of SnS2 Nanoparticles for Adsorbent Application
2.3. Preparation of SnS2 Thin Film for Photovoltaic Application
2.4. Characterization Details
3. Results and Discussion
3.1. SnS2 Nanoparticles for Adsorbent Application
3.1.1. Growth of SnS2 Nanoparticles
3.1.2. Phase Purity Confirmation of SnS2 Nanoparticles
3.1.3. Elemental Purity Confirmation of SnS2 Nanoparticles
3.1.4. SnS2 Nanoparticles as Adsorbent for Organic Pollutants (RhB Dye)
3.2. SnS2 Thin Films for Photovoltaic Application
3.2.1. Crystal Structure of SnS2 Thin Film
3.2.2. Optical Band Gap of SnS2 Thin Film
3.2.3. SnS2 Thin Film as a Buffer in CIGS Solar Cell
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IEA. Global Electricity Demand Is Growing Faster than Renewables, Driving Strong Increase in Generation from Fossil Fuels. Available online: https://www.iea.org/news/global-electricity-demand-is-growing-faster-than-renewables-driving-strong-increase-in-generation-from-fossil-fuels (accessed on 28 October 2021).
- CIGS-PV-Net CIGS Thin-Film Photovoltaics-News. Available online: http://cigs-pv.net/%0Ahttp://files/98/CIGS-WhitePaper.pdf%0Ahttp://files/100/cigs-pv.net.html (accessed on 28 October 2021).
- Jung, H.; Park, Y.; Gedi, S.; Reddy, V.R.M.; Ferblantier, G.; Kim, W.K. Al-doped zinc stannate films for photovoltaic applications. Korean J. Chem. Eng. 2020, 37, 730–735. [Google Scholar] [CrossRef]
- Moon, D.; Gedi, S.; Alhammadi, S.; Minnam Reddy, V.R.; Kim, W.K. Surface passivation of a Cu(In,Ga)Se2 photovoltaic absorber using a thin indium sulfide layer. Appl. Surf. Sci. 2020, 510, 145426. [Google Scholar] [CrossRef]
- Witte, W.; Spiering, S.; Hariskos, D. Substitution of the CdS buffer layer in CIGS thin-film solar cells. Vák. Forsch. Prax. 2014, 26, 23–27. [Google Scholar] [CrossRef]
- Schock, H.-W.; Noufi, R. CIGS-based solar cells for the next millennium. Prog. Photovolt. Res. Appl. 2000, 8, 151–160. [Google Scholar] [CrossRef]
- Solar Frontier Achieves World Record CIGS Thin-Film Solar Cell Efficiency of 23.35%. Available online: https://www.solar-frontier.com/eng/news/2019/0117_press.html (accessed on 30 October 2021).
- Orgassa, K.; Rau, U.; Nguyen, Q.; Werner Schock, H.; Werner, J.H. Role of the CdS buffer layer as an active optical element in Cu(In,Ga)Se2 thin-film solar cells. Prog. Photovolt. Res. Appl. 2002, 10, 457–463. [Google Scholar] [CrossRef]
- Tötsch, W. Cadmium—Towards a rational use of a toxic element. Environ. Manag. 1990, 14, 333–338. [Google Scholar] [CrossRef]
- Han, S.; Liu, K.; Hu, L.; Teng, F.; Yu, P.; Zhu, Y. Superior adsorption and regenerable dye adsorbent based on flower-like molybdenum disulfide nanostructure. Sci. Rep. 2017, 7, 43599. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, B.; Mei, D.; Zhang, H.; Liu, J. Adsorption of methyl violet from aqueous solution by halloysite nanotubes. Desalination 2011, 268, 111–116. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Hao, O.J.; Kim, H.; Chiang, P.-C. Decolorization of Wastewater. Crit. Rev. Environ. Sci. Technol. 2000, 30, 449–505. [Google Scholar] [CrossRef]
- Lei, C.; Pi, M.; Jiang, C.; Cheng, B.; Yu, J. Synthesis of hierarchical porous zinc oxide (ZnO) microspheres with highly efficient adsorption of Congo red. J. Colloid Interface Sci. 2017, 490, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhou, C.; Jin, Y.; Jing, Q.; Yang, Y.; Shen, X.; Tang, Q.; Mu, Y.; Du, A.-K. Facile synthesis of 3D porous thermally exfoliated g-C3N4 nanosheet with enhanced photocatalytic degradation of organic dye. J. Colloid Interface Sci. 2016, 468, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Bulai, I.M.; Venturino, E. Biodegradation of organic pollutants in a water body. J. Math. Chem. 2016, 54, 1387–1403. [Google Scholar] [CrossRef]
- Arshadi, M.; Mousavinia, F.; Amiri, M.J.; Faraji, A.R. Adsorption of methyl orange and salicylic acid on a nano-transition metal composite: Kinetics, thermodynamic and electrochemical studies. J. Colloid Interface Sci. 2016, 483, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Baruah, M.; Ezung, S.L.; Supong, A.; Bhomick, P.C.; Kumar, S.; Sinha, D. Synthesis, characterization of novel Fe-doped TiO2 activated carbon nanocomposite towards photocatalytic degradation of Congo red, E. coli, and S. aureus. Korean J. Chem. Eng. 2021, 38, 1277–1290. [Google Scholar] [CrossRef]
- Hu, L.; Yuan, H.; Zou, L.; Chen, F.; Hu, X. Adsorption and visible light-driven photocatalytic degradation of Rhodamine B in aqueous solutions by Ag@AgBr/SBA-15. Appl. Surf. Sci. 2015, 355, 706–715. [Google Scholar] [CrossRef]
- Wan, D.; Li, W.; Wang, G.; Chen, K.; Lu, L.; Hu, Q. Adsorption and heterogeneous degradation of rhodamine B on the surface of magnetic bentonite material. Appl. Surf. Sci. 2015, 349, 988–996. [Google Scholar] [CrossRef]
- Wang, S.; Yang, B.; Liu, Y. Synthesis of a hierarchical SnS2 nanostructure for efficient adsorption of Rhodamine B dye. J. Colloid Interface Sci. 2017, 507, 225–233. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Lindwall, G.; Shang, S.L.; Kelly, N.R.; Anderson, T.; Liu, Z.K. Thermodynamics of the S–Sn system: Implication for synthesis of earth abundant photovoltaic absorber materials. Sol. Energy 2016, 125, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, S.; Kumar, B.; Dwivedi, D.K. Numerical simulation of non-toxic In2S3/SnS2 buffer layer to enhance CZTS solar cells efficiency by optimizing device parameters. Optik 2021, 227, 166087. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, L.; Wei, Z. Photoresponsive field-effect transistors based on multilayer SnS2 nanosheets. J. Semicond. 2017, 38, 34001. [Google Scholar] [CrossRef]
- Sánchez-Juárez, A.; Tiburcio-Silver, A.; Ortiz, A. Fabrication of SnS2/SnS heterojunction thin film diodes by plasma-enhanced chemical vapor deposition. Thin Solid Films 2005, 480, 452–456. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, H.; Gao, S.; Sun, Z.; Liu, Q.; Leu, Q.; Lei, F.; Yao, T.; He, J.; Wei, S.; et al. Freestanding tin disulfide single-layers realizing efficient visible-light water splitting. Angew. Chem. Int. Ed. 2012, 51, 8727–8731. [Google Scholar] [CrossRef]
- Ou, J.Z.; Ge, W.; Carey, B.; Daeneke, T.; Rotbart, A.; Shan, W.; Wang, Y.; Fu, Z.; Chrimes, A.F.; Wlodarski, W.; et al. Physisorption-based charge transfer in two-dimensional SnS2 for selective and reversible NO2 gas sensing. ACS Nano 2015, 9, 10313–10323. [Google Scholar] [CrossRef]
- Gedi, S.; Minna Reddy, V.R.; Pejjai, B.; Jeon, C.-W.; Park, C.; Ramakrishna Reddy, K.T. A facile inexpensive route for SnS thin film solar cells with SnS2 buffer. Appl. Surf. Sci. 2016, 372, 116–124. [Google Scholar] [CrossRef]
- Minnam Reddy, V.R.; Lindwall, G.; Pejjai, B.; Gedi, S.; Kotte, T.R.R.; Sugiyama, M.; Liu, Z.K.; Park, C. α-SnSe thin film solar cells produced by selenization of magnetron sputtered tin precursors. Sol. Energy Mater. Sol. Cells 2018, 176, 251–258. [Google Scholar] [CrossRef]
- Gedi, S.; Minnam Reddy, V.R.; Pejjai, B.; Park, C.; Jeon, C.W.; Kotte, T.R.R. Studies on chemical bath deposited SnS2 films for Cd-free thin film solar cells. Ceram. Int. 2017, 43, 3713–3719. [Google Scholar] [CrossRef]
- Wu, Z.; Xue, Y.; Zhang, Y.; Li, J.; Chen, T. SnS2 nanosheet-based microstructures with high adsorption capabilities and visible light photocatalytic activities. RSC Adv. 2015, 5, 24640–24648. [Google Scholar] [CrossRef]
- Matyszczak, G.; Fidler, A.; Polesiak, E.; Sobieska, M.; Morawiec, K.; Zajkowska, W.; Lawniczak-Jablonska, K.; Kuzmiuk, P. Application of sonochemically synthesized SnS and SnS2 in the electro-Fenton process: Kinetics and enhanced decolorization. Ultrason. Sonochemistry 2020, 68, 105186. [Google Scholar] [CrossRef]
- Crist, B.V.; Crisst, D.B.V. Handbook of Monochromatic XPS Spectra; Wiley: New York, NY, USA, 2000; Volume 1. [Google Scholar]
- Lather, R.; Jeevanandam, P. Synthesis of Zn2+ doped SnS2 nanoparticles using a novel thermal decomposition approach and their application as adsorbent. J. Alloy. Compd. 2022, 891, 161989. [Google Scholar] [CrossRef]
- Bayati, M.R.; Golestani-Fard, F.; Moshfegh, A.Z. Visible photodecomposition of methylene blue over micro arc oxidized WO3–loaded TiO2 nano-porous layers. Appl. Catal. A Gen. 2010, 382, 322–331. [Google Scholar] [CrossRef]
- Du, W.; Deng, D.; Han, Z.; Xiao, W.; Bian, C.; Qian, X. Hexagonal tin disulfide nanoplatelets: A new photocatalyst driven by solar light. CrystEngComm 2011, 13, 2071. [Google Scholar] [CrossRef]
- Singh, K.P.; Gupta, S.; Singh, A.K.; Sinha, S. Experimental design and response surface modeling for optimization of Rhodamine B removal from water by magnetic nanocomposite. Chem. Eng. J. 2010, 165, 151–160. [Google Scholar] [CrossRef]
- Jinendra, U.; Bilehal, D.; Nagabhushana, B.M.; Kumar, A.P. Adsorptive removal of Rhodamine B dye from aqueous solution by using graphene–based nickel nanocomposite. Heliyon 2021, 7, e06851. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Cao, L.; Lu, L. Magnetite/reduced graphene oxide nanocomposites: One step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano Res. 2011, 4, 550–562. [Google Scholar] [CrossRef]
- Wang, J.; Tsuzuki, T.; Tang, B.; Hou, X.; Sun, L.; Wang, X. Reduced graphene oxide/ZnO composite: Reusable adsorbent for pollutant management. ACS Appl. Mater. Interfaces 2012, 4, 3084–3090. [Google Scholar] [CrossRef]
- Oyetade, O.A.; Nyamori, V.O.; Martincigh, B.S.; Jonnalagadda, S.B. Effectiveness of carbon nanotube–cobalt ferrite nanocomposites for the adsorption of rhodamine B from aqueous solutions. RSC Adv. 2015, 5, 22724–22739. [Google Scholar] [CrossRef]
- Kerkez, Ö.; Bayazit, Ş.S. Magnetite decorated multi-walled carbon nanotubes for removal of toxic dyes from aqueous solutions. J. Nanoparticle Res. 2014, 16, 2431. [Google Scholar] [CrossRef]
- Konicki, W.; Siber, D.; Narkiewicz, U. Removal of Rhodamine B from aqueous solution by ZnFe2O4 nanocomposite with magnetic separation performance. Pol. J. Chem. Technol. 2017, 19, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Jeong, D.; Reddy, V.R.M.; Pallavolu, M.R.; Cho, H.; Park, C. Investigation on the performance of SnS solar cells grown by sputtering and effusion cell evaporation. Korean J. Chem. Eng. 2020, 37, 1066–1070. [Google Scholar] [CrossRef]
- Alhammadi, S.; Jung, H.; Kwon, S.; Park, H.; Shim, J.-J.; Cho, M.H.; Lee, M.; Kim, J.S.; Kim, W.K. Effect of Gallium doping on CdS thin film properties and corresponding Cu(InGa)Se2/CdS:Ga solar cell performance. Thin Solid Films 2018, 660, 207–212. [Google Scholar] [CrossRef]
- Park, Y.; Ferblantier, G.; Slaoui, A.; Dinia, A.; Park, H.; Alhammadi, S.; Kim, W.K. Yb-doped zinc tin oxide thin film and its application to Cu(InGa)Se2 solar cells. J. Alloys Compd. 2020, 815, 152360. [Google Scholar] [CrossRef]
- Alhammadi, S.; Moon, K.; Park, H.; Kim, W.K. Effect of different cadmium salts on the properties of chemical-bath-deposited CdS thin films and Cu(InGa)Se2 solar cells. Thin Solid Films 2017, 625, 56–61. [Google Scholar] [CrossRef]
- Cho, K.S.; Jang, J.; Park, J.H.; Lee, D.-K.; Song, S.; Kim, K.; Eo, Y.-J.; Yun, J.H.; Gwak, J.; Chung, C.-H. Optimal CdS buffer thickness to form high-quality CdS/Cu(In,Ga)Se2 junctions in solar cells without plasma damage and shunt paths. ACS Omega 2020, 5, 23983–23988. [Google Scholar] [CrossRef]
- Ghorpade, U.; Suryawanshi, M.; Shin, S.W.; Gurav, K.; Patil, P.; Pawar, S.; Hong, C.W.; Kim, J.H.; Kolekar, S. Towards environmentally benign approaches for the synthesis of CZTSSe nanocrystals by a hot injection method: A status review. Chem. Commun. 2014, 50, 11258. [Google Scholar] [CrossRef]









| Adsorbent | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|
| Fe3O4/carbon nanocomposite | 29.48 | [38] |
| Ni/rGO nanocomposite | 65.31 | [39] |
| Fe3O4/rGO nanocomposite | 13.15 | [40] |
| ZnO/rGO nanocomposites | 32.6 | [41] |
| CoFe2O4/MWCNT nanocomposites | 35.91 | [42] |
| CoFe2O4 NPs | 5.17 | |
| Fe3O4/MWCNT nanocomposites | 11.44 | [43] |
| ZnFe2O4 NPs | 12.1 | [44] |
| SnS2 NPs | 21.25 | Present work |
| Solar Cell Performance Parameters | Type of Buffer Layer | |
|---|---|---|
| CIGS/CdS | CIGS/SnS2 | |
| Short circuit current density, JSC (mAcm−2) | 27.1 | 25.7 |
| Open-circuit voltage, VOC (V) | 0.51 | 0.41 |
| Fill factor, FF (%) | 53.8 | 49.0 |
| Efficiency, η (%) | 7.5 | 5.1 |
| Shunt resistance, RSh (Ω cm2) | 929 | 255 |
| Series resistance, RS (Ω cm2) | 14.5 | 13.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gedi, S.; Alhammadi, S.; Noh, J.; Minnam Reddy, V.R.; Park, H.; Rabie, A.M.; Shim, J.-J.; Kang, D.; Kim, W.K. SnS2 Nanoparticles and Thin Film for Application as an Adsorbent and Photovoltaic Buffer. Nanomaterials 2022, 12, 282. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12020282
Gedi S, Alhammadi S, Noh J, Minnam Reddy VR, Park H, Rabie AM, Shim J-J, Kang D, Kim WK. SnS2 Nanoparticles and Thin Film for Application as an Adsorbent and Photovoltaic Buffer. Nanomaterials. 2022; 12(2):282. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12020282
Chicago/Turabian StyleGedi, Sreedevi, Salh Alhammadi, Jihyeon Noh, Vasudeva Reddy Minnam Reddy, Hyeonwook Park, Abdelrahman Mohamed Rabie, Jae-Jin Shim, Dohyung Kang, and Woo Kyoung Kim. 2022. "SnS2 Nanoparticles and Thin Film for Application as an Adsorbent and Photovoltaic Buffer" Nanomaterials 12, no. 2: 282. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12020282