Oxygen-Sensitive Photo- and Radioluminescent Polyurethane Nanoparticles Modified with Octahedral Iodide Tungsten Clusters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of (Choline)2[{W6I8}I6]
2.2. Synthesis of Polyurethane Nanosized Particles
2.3. Singlet Oxygen Generation
2.4. Stern–Volmer Dependence
2.5. XEOL
3. Results and Discussion
3.1. Synthesis and Characterization
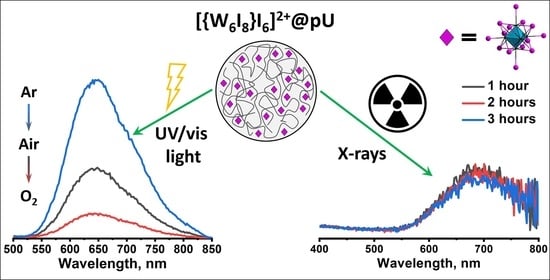
3.2. Photoluminescence
3.3. O2 Generation
3.4. Oxygen Sensing and Stability in Water
3.5. X-ray-Excited Optical Luminescence (XEOL)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent Progress in Photosensitizers for Overcoming the Challenges of Photodynamic Therapy: From Molecular Design to Application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.C.; Nguyen, V.-N.; Choi, Y.; Lee, S.; Yoon, J. Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, R.; Lee, J.; Yang, S.-G. Clinical Development of Photodynamic Agents and Therapeutic Applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Dave, D.; Desai, U.; Deshpande, N. Photodynamic Therapy: A View through Light. J. Orofac. Res. 2012, 2, 82–86. [Google Scholar] [CrossRef]
- Belanova, A.; Chmykhalo, V.; Beseda, D.; Belousova, M.; Butova, V.; Soldatov, A.; Makarenko, Y.; Zolotukhin, P. A Mini-Review of X-ray Photodynamic Therapy (XPDT) Nonoagent Constituents’ Safety and Relevant Design Considerations. Photochem. Photobiol. Sci 2020, 19, 1134–1144. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J. Using Nanoparticles to Enable Simultaneous Radiation and Photodynamic Therapies for Cancer Treatment. J. Nanosci. Nanotechnol. 2006, 6, 1159–1166. [Google Scholar] [CrossRef]
- Wang, G.D.; Nguyen, H.T.; Chen, H.; Cox, P.B.; Wang, L.; Nagata, K.; Hao, Z.; Wang, A.; Li, Z.; Xie, J. X-ray Induced Photodynamic Therapy: A Combination of Radiotherapy and Photodynamic Therapy. Theranostics 2016, 6, 2295–2305. [Google Scholar] [CrossRef]
- Gadzhimagomedova, Z.; Zolotukhin, P.; Kit, O.; Kirsanova, D.; Soldatov, A. Nanocomposites for X-ray Photodynamic Therapy. Int. J. Mol. Sci. 2020, 21, 4004. [Google Scholar] [CrossRef]
- Park, W.; Cho, S.; Kang, D.; Han, J.-H.; Park, J.-H.; Lee, B.; Lee, J.; Kim, D.-H. Tumor Microenvironment Targeting Nano–Bio Emulsion for Synergistic Combinational X-ray PDT with Oncolytic Bacteria Therapy. Adv. Healthc. Mater. 2020, 9, 1901812. [Google Scholar] [CrossRef]
- Kirakci, K.; Kubát, P.; Fejfarová, K.; Martinčík, J.; Nikl, M.; Lang, K. X-ray Inducible Luminescence and Singlet Oxygen Sensitization by an Octahedral Molybdenum Cluster Compound: A New Class of Nanoscintillators. Inorg. Chem. 2016, 55, 803–809. [Google Scholar] [CrossRef]
- Kirakci, K.; Pozmogova, T.N.; Protasevich, A.Y.; Vavilov, G.D.; Stass, D.V.; Shestopalov, M.A.; Lang, K. A Water-Soluble Octahedral Molybdenum Cluster Complex as a Potential Agent for X-Ray Induced Photodynamic Therapy. Biomater. Sci. 2021, 9, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Pozmogova, T.N.; Sitnikova, N.A.; Pronina, E.V.; Miroshnichenko, S.M.; Kushnarenko, A.O.; Solovieva, A.O.; Bogachev, S.S.; Vavilov, G.D.; Efremova, O.A.; Vorotnikov, Y.A.; et al. Hybrid System {W6I8}-cluster/dsDNA as an Agent For Targeted X-Ray Induced Photodynamic Therapy of Cancer Stem Cells. Mater. Chem. Front. 2021, 5, 7499–7507. [Google Scholar] [CrossRef]
- Evtushok, D.V.; Melnikov, A.R.; Vorotnikova, N.A.; Vorotnikov, Y.A.; Ryadun, A.A.; Kuratieva, N.V.; Kozyr, K.V.; Obedinskaya, N.R.; Kretov, E.I.; Novozhilov, I.N.; et al. A Comparative Study of Optical Properties and X-Ray Induced Luminescence of Octahedral Molybdenum and Tungsten Cluster Complexes. Dalton Trans. 2017, 46, 11738–11747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, J.A.; Turro, C.; Newsham, M.D.; Nocera, D.G. Oxygen Quenching of Electronically Excited Hexanuclear Molybdenum and Tungsten Halide Clusters. J. Phys. Chem. 1990, 94, 4500–4507. [Google Scholar] [CrossRef]
- Riehl, L.; Seyboldt, A.; Ströbele, M.; Enseling, D.; Jüstel, T.; Westberg, M.; Ogilby, P.R.; Meyer, H.J. A Ligand Substituted Tungsten Iodide Cluster: Luminescence vs. Singlet Oxygen Production. Dalton Trans. 2016, 45, 15500–15506. [Google Scholar] [CrossRef] [Green Version]
- Mikhaylov, M.A.; Sokolov, M.N. Molybdenum Iodides—From Obscurity to Bright Luminescence. Eur. J. Inorg. Chem. 2019, 2019, 4181–4197. [Google Scholar] [CrossRef]
- Petunin, A.A.; Evtushok, D.V.; Vorotnikova, N.A.; Kuratieva, N.V.; Vorotnikov, Y.A.; Shestopalov, M.A. Hexasubstituted Iodide Tungsten Cluster Complexes with Azide and Isothiocyanate Ligands. Eur. J. Inorg. Chem. 2020, 2020, 2177–2181. [Google Scholar] [CrossRef]
- Vorotnikova, N.A.; Alekseev, A.Y.; Vorotnikov, Y.A.; Evtushok, D.V.; Molard, Y.; Amela-Cortes, M.; Cordier, S.; Smolentsev, A.I.; Burton, C.G.; Kozhin, P.M.; et al. Octahedral Molybdenum Cluster as a Photoactive Antimicrobial Additive to a Fluoroplastic. Mater. Sci. Eng. C 2019, 105, 110150. [Google Scholar] [CrossRef]
- Hummel, T.; Dutczak, D.; Alekseev, A.Y.; Adamenko, L.S.; Shestopalov, M.A.; Mironov, Y.V.; Enseling, D.; Jüstel, T.; Meyer, H.-J. Photodynamic Properties of Tungsten Iodide Clusters Incorporated into Silicone: A2[M6I8L6]@silicone. RSC Adv. 2020, 10, 22257–22263. [Google Scholar] [CrossRef]
- Vorotnikova, N.A.; Bardin, V.A.; Vorotnikov, Y.A.; Kirakci, K.; Adamenko, L.S.; Alekseev, A.Y.; Meyer, H.J.; Kubát, P.; Mironov, Y.V.; Lang, K.; et al. Heterogeneous Photoactive Antimicrobial Coatings Based on a Fluoroplastic Doped with an Octahedral Molybdenum Cluster Compound. Dalton Trans. 2021, 50, 8467–8475. [Google Scholar] [CrossRef]
- Svezhentseva, E.V.; Vorotnikov, Y.A.; Solovieva, A.O.; Pozmogova, T.N.; Eltsov, I.V.; Ivanov, A.A.; Evtushok, D.V.; Miroshnichenko, S.M.; Yanshole, V.V.; Eling, C.J.; et al. From Photoinduced to Dark Cytotoxicity through an Octahedral Cluster Hydrolysis. Chem. Eur. J. 2018, 24, 17915–17920. [Google Scholar] [CrossRef] [PubMed]
- Pronina, E.V.; Pozmogova, T.N.; Vorotnikov, Y.A.; Ivanov, A.A.; Shestopalov, M.A. The Role of Hydrolysis in Biological Effects of Molybdenum Cluster with DMSO Ligands. J. Biol. Inorg. Chem. 2022, 27, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Efremova, O.A.; Shestopalov, M.A.; Chirtsova, N.A.; Smolentsev, A.I.; Mironov, Y.V.; Kitamura, N.; Brylev, K.A.; Sutherland, A.J. A Highly Emissive Inorganic Hexamolybdenum Cluster Complex as a Handy Precursor for the Preparation of New Luminescent Materials. Dalton Trans. 2014, 43, 6021–6025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorotnikova, N.A.; Efremova, O.A.; Tsygankova, A.R.; Brylev, K.A.; Edeleva, M.V.; Kurskaya, O.G.; Sutherland, A.J.; Shestopalov, A.M.; Mironov, Y.V.; Shestopalov, M.A. Characterization and Cytotoxicity Studies of Thiol-Modified Polystyrene Microbeads Doped with [{Mo6X8}(NO3)6]2− (X = Cl, Br, I). Polym. Adv. Technol. 2016, 27, 922–928. [Google Scholar] [CrossRef] [Green Version]
- Felip-León, C.; Arnau Del Valle, C.; Pérez-Laguna, V.; Isabel Millán-Lou, M.; Miravet, J.F.; Mikhailov, M.; Sokolov, M.N.; Rezusta-López, A.; Galindo, F. Superior Performance of Macroporous over Gel Type Polystyrene as a Support for the Development of Photo-Bactericidal Materials. J. Mater. Chem. B 2017, 5, 6058–6064. [Google Scholar] [CrossRef]
- Svezhentseva, E.V.; Solovieva, A.O.; Vorotnikov, Y.A.; Kurskaya, O.G.; Brylev, K.A.; Tsygankova, A.R.; Edeleva, M.V.; Gyrylova, S.N.; Kitamura, N.; Efremova, O.A.; et al. Water-Soluble Hybrid Materials Based on {Mo6X8}4+ (X = Cl, Br, I) Cluster Complexes and Sodium Polystyrene Sulfonate. New J. Chem. 2017, 41, 1670–1676. [Google Scholar] [CrossRef] [Green Version]
- Brandhonneur, N.; Boucaud, Y.; Verger, A.; Dumait, N.; Molard, Y.; Cordier, S.; Dollo, G. Molybdenum Cluster Loaded PLGA Nanoparticles as Efficient Tools Against Epithelial Ovarian Cancer. Int. J. Pharm. 2021, 592, 120079. [Google Scholar] [CrossRef]
- Dollo, G.; Boucaud, Y.; Amela-Cortes, M.; Molard, Y.; Cordier, S.; Brandhonneur, N. PLGA Nanoparticles Embedding Molybdenum Cluster Salts: Influence of Chemical Composition on Physico-Chemical Properties, Encapsulation Efficiencies, Colloidal Stabilities and in vitro Release. Int. J. Pharm. 2020, 576, 119025. [Google Scholar] [CrossRef]
- Semsarzadeh, M.A.; Sadeghi, M.; Barikani, M. Effect of Chain Extender Length on Gas Permeation Properties of Polyurethane Membranes. Iran. Polym. J. 2008, 17, 431–440. [Google Scholar]
- Jiang, X.; Ding, J.; Kumar, A. Polyurethane–poly(vinylidene fluoride) (PU–PVDF) Thin Film Composite Membranes for Gas Separation. J. Membr. Sci. 2008, 323, 371–378. [Google Scholar] [CrossRef] [Green Version]
- Morral-Ruíz, G.; Melgar-Lesmes, P.; Solans, C.; García-Celma, M.J. Polyurethane Nanoparticles, a New Tool for Biomedical Applications? In Advances in Polyurethane Biomaterials; Cooper, S.L., Guan, J., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 195–216. [Google Scholar] [CrossRef]
- Sask, K.N.; Berry, L.R.; Chan, A.K.C.; Brash, J.L. Modification of Polyurethane Surface with an Antithrombin–Heparin Complex for Blood Contact: Influence of Molecular Weight of Polyethylene Oxide Used as a Linker/Spacer. Langmuir 2012, 28, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Noorisafa, F.; Razmjou, A.; Emami, N.; Low, Z.-X.; Korayem, A.H.; Kajani, A.A. Surface Modification of Polyurethane via Creating a Biocompatible Superhydrophilic Nanostructured Layer: Role of Surface Chemistry and Structure. J. Exp. Nanosci. 2016, 11, 1087–1109. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, C. Polyurethane for Biomedical Applications: A Review of Recent Developments. In The Design and Manufacture of Medical Devices; Davim, J.P., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 115–151. [Google Scholar] [CrossRef]
- Lu, F.; Li, Z.; Kang, Y.; Su, Z.; Yu, R.; Zhang, S. Black Phosphorus Quantum Dots Encapsulated in Anionic Waterborne Polyurethane Nanoparticles for Enhancing Stability and Reactive Oxygen Species Generation for Cancer PDT/PTT Therapy. J. Mater. Chem. B 2020, 8, 10650–10661. [Google Scholar] [CrossRef]
- Amela-Cortes, M.; Paofai, S.; Cordier, S.; Folliot, H.; Molard, Y. Tuned Red NIR Phosphorescence of Polyurethane Hybrid Composites Embedding Metallic Nanoclusters for Oxygen Sensing. Chem. Commun. 2015, 51, 8177–8180. [Google Scholar] [CrossRef]
- Loulergue, P.; Amela-Cortes, M.; Cordier, S.; Molard, Y.; Lemiègre, L.; Audic, J.L. Polyurethanes Prepared from Cyclocarbonated Broccoli Seed Oil (Pucc): New Biobased Organic Matrices for Incorporation of Phosphorescent Metal Nanocluster. J. Appl. Polym. Sci. 2017, 134, 45339. [Google Scholar] [CrossRef] [Green Version]
- Vorotnikov, Y.A.; Mikhailov, M.A.; Brylev, K.A.; Piryazev, D.A.; Kuratieva, N.V.; Sokolov, M.N.; Mironov, Y.V.; Shestopalov, M.A. Synthesis, Crystal Structure, and Luminescence Properties of Complexes (4-ViBnNMe3)2[{M6(µ3-I)8}I6] (M = Mo, W; (4-ViBnNMe3)+ is Trimethyl(4-vinylbenzyl)ammonium). Russ. Chem. Bull. 2015, 64, 2591–2596. [Google Scholar] [CrossRef]
- Kalneus, E.V.; Melnikov, A.R.; Korolev, V.V.; Ivannikov, V.I.; Stass, D.V. A Low-Field Magnetically Affected Reaction Yield (MARY) Spectrometer with Spectral Fluorescence Resolution. Appl. Magn. Reson. 2013, 44, 81–96. [Google Scholar] [CrossRef]
- Stass, D.V.; Vorotnikova, N.A.; Shestopalov, M.A. Direct Observation of X-ray Excited Optical Luminescence from a Re6 Metal Cluster in True Aqueous Solution: The Missing Link Between Material Characterization and in Vivo Applications. J. Appl. Phys. 2021, 129, 183102. [Google Scholar] [CrossRef]
- Melnikov, A.R.; Kalneus, E.V.; Korolev, V.V.; Dranov, I.G.; Kruppa, A.I.; Stass, D.V. Highly Efficient Exciplex Formation via Radical Ion Pair Recombination in X-Irradiated Alkane Solutions for Luminophores with Short Fluorescence Lifetimes. Photochem. Photobiol. Sci. 2014, 13, 1169–1179. [Google Scholar] [CrossRef]
- Kobzeva, T.V.; Melnikov, A.R.; Karogodina, T.Y.; Zikirin, S.B.; Stass, D.V.; Molin, Y.N.; Rodicheva, E.K.; Medvedeva, S.E.; Puzyr, A.P.; Burov, A.A.; et al. Stimulation of Luminescence of Mycelium of Luminous Fungus Neonothopanus Nambi by Ionizing Radiation. Luminescence 2014, 29, 703–710. [Google Scholar] [CrossRef]
- Luo, S.-G.; Tan, H.-M.; Zhang, J.-G.; Wu, Y.-J.; Pei, F.-K.; Meng, X.-H. Catalytic Mechanisms of Triphenyl Bismuth, Dibutyltin Dilaurate, and Their Combination in Polyurethane-Forming Reaction. J. Appl. Polym. Sci. 1997, 65, 1217–1225. [Google Scholar] [CrossRef]
- Alfani, R.; Iannace, S.; Nicolais, L. Synthesis and Characterization of Starch-Based Polyurethane Foams. J. Appl. Polym. Sci. 1998, 68, 739–745. [Google Scholar] [CrossRef]
- Alferiev, I.S. Novel Elastomeric Polyurethanes with Pendant Epoxy Groups as Highly Reactive Auxiliary Groups for Further Derivatizations. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 4378–4385. [Google Scholar] [CrossRef]
- Alferiev, I.S.; Fishbein, I. Activated Polyurethane Modified with Latent Thiol Groups. Biomaterials 2002, 23, 4753–4758. [Google Scholar] [CrossRef]
- Badri, K.B.H.; Wong, C.S.; Maisara, S.B.R.S.; Liow, C.H.; Norhafiza, Y.B.; Nor, R.A.N. FTIR Spectroscopy Analysis of the Prepolymerization of Palm-Based Polyurethane. Solid State Sci. Technol. 2010, 18, 1–8. [Google Scholar]
- Asefnejad, A.; Khorasani, M.T.; Behnamghader, A.; Farsadzadeh, B.; Bonakdar, S. Manufacturing of Biodegradable Polyurethane Scaffolds Based on Polycaprolactone Using a Phase Separation Method: Physical Properties and In Vitro Assay. Int. J. Nanomed. 2011, 6, 2375–2384. [Google Scholar] [CrossRef] [Green Version]
- Costuas, K.; Garreau, A.; Bulou, A.; Fontaine, B.; Cuny, J.; Gautier, R.; Mortier, M.; Molard, Y.; Duvail, J.L.; Faulques, E.; et al. Combined Theoretical and Time-Resolved Photoluminescence Investigations of [Mo6Bri8Bra6]2− Metal Cluster Units: Evidence of Dual Emission. Phys. Chem. Chem. Phys. 2015, 17, 28574–28585. [Google Scholar] [CrossRef]
- Kitamura, N.; Kuwahara, Y.; Ueda, Y.; Ito, Y.; Ishizaka, S.; Sasaki, Y.; Tsuge, K.; Akagi, S. Excited Triplet States of [{Mo6Cl8}Cl6]2−, [{Re6S8}Cl6]4−, and [{W6Cl8}Cl6]2− Clusters. Bull. Chem. Soc. Jpn. 2017, 90, 1164–1173. [Google Scholar] [CrossRef]
- Kirakci, K.; Kubát, P.; Langmaier, J.; Polívka, T.; Fuciman, M.; Fejfarová, K.; Lang, K. A Comparative Study of the Redox and Excited State Properties of (nBu4N)2[Mo6X14] and (nBu4N)2[Mo6X8(CF3COO)6] (X = Cl, Br, or I). Dalton Trans. 2013, 42, 7224–7232. [Google Scholar] [CrossRef]
- Ossola, R.; Jönsson, O.M.; Moor, K.; McNeill, K. Singlet Oxygen Quantum Yields in Environmental Waters. Chem. Rev. 2021, 121, 4100–4146. [Google Scholar] [CrossRef]
- Seyboldt, A.; Enseling, D.; Jüstel, T.; Ivanović, M.; Peisert, H.; Chassé, T.; Meyer, H.-J. Ligand Influence on the Photophysical Properties and Electronic Structures of Tungsten Iodide Clusters. Eur. J. Inorg. Chem. 2017, 2017, 5387–5394. [Google Scholar] [CrossRef]
- Kirakci, K.; Zelenka, J.; Rumlová, M.; Cvačka, J.; Ruml, T.; Lang, K. Cationic Octahedral Molybdenum Cluster Complexes Functionalized with Mitochondria-Targeting Ligands: Photodynamic Anticancer and Antibacterial Activities. Biomater. Sci. 2019, 7, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Marchuk, M.V.; Vorotnikova, N.A.; Vorotnikov, Y.A.; Kuratieva, N.V.; Stass, D.V.; Shestopalov, M.A. Optical Property Trends in a Family of {Mo6I8} Aquahydroxo Complexes. Dalton Trans. 2021, 50, 8794–8802. [Google Scholar] [CrossRef] [PubMed]
- Lindig, B.A.; Rodgers, M.A.J.; Schaap, A.P. Determination of the Lifetime of Singlet Oxygen in Water-D2 Using 9,10-Anthracenedipropionic Acid, a Water-Soluble Probe. J. Am. Chem. Soc. 1980, 102, 5590–5593. [Google Scholar] [CrossRef]







| Sample | Intensity Loss, % | Rate Constant, min−1 | |
|---|---|---|---|
| 2nd 3 Scans | 3rd 3 Scans | ||
| (Bu4N)2[{W6I8}I6] | 6% | 11% | 0.0011 |
| 1 | 32% | 53% | 0.01 |
| 10.5@pU solid | 8% | 16% | 0.0019 |
| 10.5@pU in D2O | 0% | 0% | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardin, V.A.; Vorotnikov, Y.A.; Stass, D.V.; Vorotnikova, N.A.; Shestopalov, M.A. Oxygen-Sensitive Photo- and Radioluminescent Polyurethane Nanoparticles Modified with Octahedral Iodide Tungsten Clusters. Nanomaterials 2022, 12, 3580. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12203580
Bardin VA, Vorotnikov YA, Stass DV, Vorotnikova NA, Shestopalov MA. Oxygen-Sensitive Photo- and Radioluminescent Polyurethane Nanoparticles Modified with Octahedral Iodide Tungsten Clusters. Nanomaterials. 2022; 12(20):3580. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12203580
Chicago/Turabian StyleBardin, Vyacheslav A., Yuri A. Vorotnikov, Dmitri V. Stass, Natalya A. Vorotnikova, and Michael A. Shestopalov. 2022. "Oxygen-Sensitive Photo- and Radioluminescent Polyurethane Nanoparticles Modified with Octahedral Iodide Tungsten Clusters" Nanomaterials 12, no. 20: 3580. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12203580