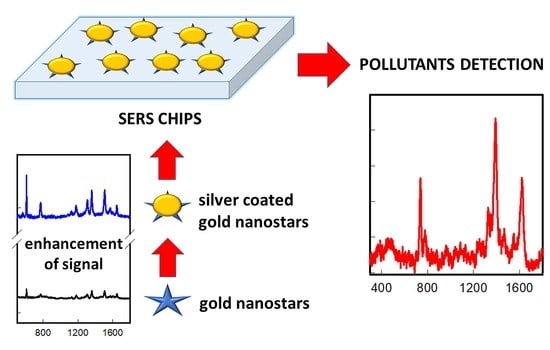
Surface-Enhanced Raman Spectroscopy Chips Based on Silver Coated Gold Nanostars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instruments
2.3. Synthesis of Gold Nanostars (GNSs)
2.4. Silver Coating of Gold Nanostars (GNS@Ag)
2.5. SERS Testing of GNS and GNS@Ag Colloidal Suspensions
2.6. Preparation of SERS-Chips
2.7. Detection of Pollutants in Water
2.8. Detection of Thiram in Ethanol
2.9. Coating of GNS@Ag Monolayers with PDMS
2.10. Detection of Toluene
3. Results and Discussion
3.1. Preparation and Optimization of GNS@Ag
3.1.1. Preparation of GNS@Ag
3.1.2. Theoretical Modelling
3.2. Preparation and Characterization of GNS@Ag Based SERS Chips
3.3. Norfloxacin Detection
3.4. Thiram Detection
3.5. Toluene Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tan, S.H.; Yougbaré, S.; Tao, H.Y.; Chang, C.C.; Kuo, T.R. Plasmonic Gold Nanoisland Film for Bacterial Theranostics. Nanomaterials 2021, 11, 3139. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.B.; Maillard, J.Y.; Simões, L.C.; Simões, M. Emerging Contaminants Affect the Microbiome of Water Systems—Strategies for Their Mitigation. NPJ Clean Water 2020, 3, 39. [Google Scholar] [CrossRef]
- Salimi, M.; Esrafili, A.; Gholami, M.; Jonidi Jafari, A.; Rezaei Kalantary, R.; Farzadkia, M.; Kermani, M.; Sobhi, H.R. Contaminants of Emerging Concern: A Review of New Approach in AOP Technologies. Environ. Monit. Assess. 2017, 189, 414. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Guo, Q.; Xu, M.; Yuan, Y.; Yao, J. Improving the SERS Detection Sensitivity of Aromatic Molecules by a PDMS-Coated Au Nanoparticle Monolayer Film. RSC Adv. 2015, 5, 53306–53312. [Google Scholar] [CrossRef]
- Wirtz, V.J.; Dreser, A.; Gonzales, R. Trends in Antibiotic Utilization in Eight Latin American Countries, 1997–2007. Rev. Panam. Salud Publica/Pan Am. J. Public Health 2010, 27, 219–225. [Google Scholar] [CrossRef]
- Jojoa-Sierra, S.D.; Silva-Agredo, J.; Herrera-Calderon, E.; Torres-Palma, R.A. Elimination of the Antibiotic Norfloxacin in Municipal Wastewater, Urine and Seawater by Electrochemical Oxidation on IrO2 Anodes. Sci. Total Environ. 2017, 575, 1228–1238. [Google Scholar] [CrossRef]
- Andreozzi, R.; Marotta, R.; Paxéus, N. Pharmaceuticals in STP Effluents and Their Solar Photodegradation in Aquatic Environment. Chemosphere 2003, 50, 1319–1330. [Google Scholar] [CrossRef]
- Usman, M.; Guo, X.; Wu, Q.; Barman, J.; Su, S.; Huang, B.; Biao, T.; Zhang, Z.; Zhan, Q. Facile Silicone Oil-Coated Hydrophobic Surface for Surface Enhanced Raman Spectroscopy of Antibiotics. RSC Adv. 2019, 9, 14109–14115. [Google Scholar] [CrossRef] [Green Version]
- Teng, P.; Gao, D.; Yang, X.; Luo, M.; Kong, D.; Gao, S.; Liu, Z.; Li, Z.; Wen, X.; Yuan, L.; et al. In Situ SERS Detection of Quinolone Antibiotic Residues in a Water Environment Based on Optofluidic In-Fiber Integrated Ag Nanoparticles. Appl. Opt. 2021, 60, 6659–6664. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W.; Zhou, S.N.; Wang, D.M.; Gong, Z.J.; Fan, M.K. Free-Standing Membrane Liquid-State Platform for SERS-Based Determination of Norfloxacin in Environmental Samples. J. Anal. Test. 2021, 5, 217–224. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, Z.; Chen, M.; Zhou, X.; Chen, W. Microwave-Assisted Synthesis of Polyamine-Functionalized Carbon Dots from Xylan and Their Use for the Detection of Tannic Acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 301–308. [Google Scholar] [CrossRef]
- Lu, S.; You, T.; Yang, N.; Gao, Y.; Yin, P. Flexible SERS Substrate Based on Ag Nanodendrite–Coated Carbon Fiber Cloth: Simultaneous Detection for Multiple Pesticides in Liquid Droplet. Anal. Bioanal. Chem. 2020, 412, 1159–1167. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Q.; Kutsanedzie, F.Y.H.; Yang, M.; Ouyang, Q.; Jiang, H. Highly Sensitive and Label-Free Determination of Thiram Residue Using Surface-Enhanced Raman Spectroscopy (SERS) Coupled with Paper-Based Microfluidics. Anal. Methods 2017, 9, 6186–6193. [Google Scholar] [CrossRef]
- Pastorello, M.; Sigoli, F.A.; dos Santos, D.P.; Mazali, I.O. On the Use of Au@Ag Core-Shell Nanorods for SERS Detection of Thiram Diluted Solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 231, 118113–118122. [Google Scholar] [CrossRef]
- D’Agostino, A.; Giovannozzi, A.M.; Mandrile, L.; Sacco, A.; Rossi, A.M.; Taglietti, A. In Situ Seed-Growth Synthesis of Silver Nanoplates on Glass for the Detection of Food Contaminants by Surface Enhanced Raman Scattering. Talanta 2020, 216, 120936–120943. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, S.; Li, X.; Zhang, Q. Determination of Thiram in Wheat Flour and Flour Improvers by High Performance Liquid Chromatography-Diode Array Detection. Chin. J. Chromatogr. 2021, 39, 652–658. [Google Scholar] [CrossRef]
- Fales, A.M.; Vo-Dinh, T. Silver Embedded Nanostars for SERS with Internal Reference (SENSIR). J. Mater. Chem. C 2015, 3, 7319–7324. [Google Scholar] [CrossRef]
- Vo-Dinh, T.; Fales, A.M.; Griffin, G.D.; Khoury, C.G.; Liu, Y.; Ngo, H.; Norton, S.J.; Register, J.K.; Wang, H.N.; Yuan, H. Plasmonic Nanoprobes: From Chemical Sensing to Medical Diagnostics and Therapy. Nanoscale 2013, 5, 10127–10140. [Google Scholar] [CrossRef]
- Nalbant Esenturk, E.; Hight Walker, A.R. Surface-Enhanced Raman Scattering Spectroscopy via Gold Nanostars. J. Raman Spectrosc. 2009, 40, 86–91. [Google Scholar] [CrossRef]
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Guerrero-Martínez, A.; Barbosa, S.; Pastoriza-Santos, I.; Liz-Marzán, L.M. Nanostars Shine Bright for You. Colloidal Synthesis, Properties and Applications of Branched Metallic Nanoparticles. Curr. Opin. Colloid Interface Sci. 2011, 16, 118–127. [Google Scholar] [CrossRef]
- Bassi, B.; Albini, B.; D’Agostino, A.; Dacarro, G.; Pallavicini, P.; Galinetto, P.; Taglietti, A. Robust, Reproducible, Recyclable SERS Substrates: Monolayers of Gold Nanostars Grafted on Glass and Coated with a Thin Silica Layer. Nanotechnology 2019, 30, 025302–025312. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, A.; Wang, Y.; Liz-Marzán, L.M. Recent Approaches toward Creation of Hot Spots for SERS Detection. J. Photochem. Photobiol. C Photochem. Rev. 2014, 21, 2–25. [Google Scholar] [CrossRef]
- Fales, A.M.; Yuan, H.; Vo-Dinh, T. Development of Hybrid Silver-Coated Gold Nanostars for Nonaggregated Surface-Enhanced Raman Scattering. J. Phys. Chem. C 2014, 118, 3708–3715. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Strobbia, P.; Cupil-Garcia, V.; Vo-Dinh, T. Fiber-Optrode SERS Probes Using Plasmonic Silver-Coated Gold Nanostars. Sens. Actuators B Chem. 2019, 287, 95–101. [Google Scholar] [CrossRef]
- Sortur, V.; Yenagi, J.; Tonannavar, J.; Jadhav, V.B.; Kulkarni, M.V. Vibrational Assignments for 7-Methyl-4-Bromomethylcoumarin, as Aided by RHF and B3LYP/6-31G* Calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 71, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Guarino-Hotz, M.; Allen, A.C.; Wang, A.; Zou, S.; Zhang, J.Z. Near-Infrared Light Absorbing Silver-Coated Hollow Gold Nanostars for Surface-Enhanced Raman Scattering Detection of Bovine Serum Albumin Using Capping Ligand Exchange. J. Phys. Chem. C 2022, 126, 1026–1035. [Google Scholar] [CrossRef]
- Pallavicini, P.; Donà, A.; Casu, A.; Chirico, G.; Collini, M.; Dacarro, G.; Falqui, A.; Milanese, C.; Sironi, L.; Taglietti, A. Triton X-100 for Three-Plasmon Gold Nanostars with Two Photothermally Active NIR (near IR) and SWIR (Short-Wavelength IR) Channels. Chem. Commun. 2013, 49, 6265–6267. [Google Scholar] [CrossRef] [Green Version]
- Guthmuller, J.; Champagne, B. Resonance Raman Scattering of Rhodamine 6G as Calculated by Time-Dependent Density Functional Theory: Vibronic and Solvent Effects. J. Phys. Chem. A 2008, 112, 3215–3223. [Google Scholar] [CrossRef]
- Taglietti, A.; Arciola, C.R.; D’Agostino, A.; Dacarro, G.; Montanaro, L.; Campoccia, D.; Cucca, L.; Vercellino, M.; Poggi, A.; Pallavicini, P.; et al. Antibiofilm Activity of a Monolayer of Silver Nanoparticles Anchored to an Amino-Silanized Glass Surface. Biomaterials 2014, 35, 1779–1788. [Google Scholar] [CrossRef]
- Huang, Q.; Fang, C.; Muhammad, M.; Yao, G. Assessment of Norfloxacin Degradation Induced by Plasma-Produced Ozone Using Surface-Enhanced Raman Spectroscopy. Chemosphere 2020, 238, 124618–124625. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, D.; Chen, Z. Ag Dendritic Nanostructures for Rapid Detection of Thiram Based on Surface-Enhanced Raman Scattering. RSC Adv. 2015, 5, 70553–70557. [Google Scholar] [CrossRef]
- Talamona, F.; Truffi, M.; Caldarone, A.A.; Ricciardi, A.; Corsi, F.; Pellegrini, G.; Morasso, C.; Taglietti, A. Stable and Scalable SERS Tags Conjugated with Neutravidin for the Detection of Fibroblast Activation Protein (FAP) in Primary Fibroblasts. Nanotechnology 2021, 32, 295703–295712. [Google Scholar] [CrossRef]
- Seo, M.; Ha, J.W. Effective Surface-Enhanced Raman Scattering of Randomly Branched Gold Nano-Urchins with Rhodamine 6G as Raman Reporters. Microchem. J. 2018, 140, 47–51. [Google Scholar] [CrossRef]
- Reid, M.T.H.; Johnson, S.G. Efficient Computation of Power, Force, and Torque in BEM Scattering Calculations. IEEE Trans. Antennas Propag. 2015, 63, 3588–3598. [Google Scholar] [CrossRef] [Green Version]
- Johnson, P.B.; Christy, R.W. Optical Constants of the Noble Metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar] [CrossRef]
- Olmon, R.L.; Slovick, B.; Johnson, T.W.; Shelton, D.; Oh, S.H.; Boreman, G.D.; Raschke, M.B. Optical Dielectric Function of Gold. Phys. Rev. B Condens. Matter Mater. Phys. 2012, 86, 742–744. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.U.; D’Archangel, J.; Sundheimer, M.L.; Tucker, E.; Boreman, G.D.; Raschke, M.B. Optical Dielectric Function of Silver. Phys. Rev. B Condens. Matter Mater. Phys. 2015, 91, 235137–235147. [Google Scholar] [CrossRef] [Green Version]
- Tsoulos, T.V.; Atta, S.; Lagos, M.J.; Beetz, M.; Batson, P.E.; Tsilomelekis, G.; Fabris, L. Colloidal Plasmonic Nanostar Antennas with Wide Range Resonance Tunability. Nanoscale 2019, 11, 18662–18671. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoint, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Li, X.; Zhang, Y.; Chen, Q.; Ye, Z.; Alqarni, Z.; Bell, S.E.J.; Xu, Y. Towards Practical and Sustainable SERS: A Review of Recent Developments in the Construction of Multifunctional Enhancing Substrates. J. Mater. Chem. C 2021, 9, 11517–11552. [Google Scholar] [CrossRef]
- Vogel, E.; Kiefer, W. Investigation of the Metal Adsorbate Interface of the System Silver Coumarin and Silver Hydrocoumarin by Means of Surface Enhanced Raman Spectroscopy. Fresenius. J. Anal. Chem. 1998, 361, 628–630. [Google Scholar] [CrossRef]
- Grys, D.B.; Chikkaraddy, R.; Kamp, M.; Scherman, O.A.; Baumberg, J.J.; de Nijs, B. Eliminating Irreproducibility in SERS Substrates. J. Raman Spectrosc. 2021, 52, 412–419. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parmigiani, M.; Albini, B.; Pellegrini, G.; Genovesi, M.; De Vita, L.; Pallavicini, P.; Dacarro, G.; Galinetto, P.; Taglietti, A. Surface-Enhanced Raman Spectroscopy Chips Based on Silver Coated Gold Nanostars. Nanomaterials 2022, 12, 3609. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12203609
Parmigiani M, Albini B, Pellegrini G, Genovesi M, De Vita L, Pallavicini P, Dacarro G, Galinetto P, Taglietti A. Surface-Enhanced Raman Spectroscopy Chips Based on Silver Coated Gold Nanostars. Nanomaterials. 2022; 12(20):3609. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12203609
Chicago/Turabian StyleParmigiani, Miriam, Benedetta Albini, Giovanni Pellegrini, Marco Genovesi, Lorenzo De Vita, Piersandro Pallavicini, Giacomo Dacarro, Pietro Galinetto, and Angelo Taglietti. 2022. "Surface-Enhanced Raman Spectroscopy Chips Based on Silver Coated Gold Nanostars" Nanomaterials 12, no. 20: 3609. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12203609