1. Introduction
Single-walled carbon nanotubes (SWCNTs) have attracted attention for their application as a photoelectric conversion material due to their outstanding solar light absorption property [
1]. The optical absorption of semiconducting SWCNTs reveals sets of chirality-dependent absorption bands in the near-infrared and visible wavelength regions, which are labeled the first (E
11) and second (E
22) transitions, corresponding to the discrete energetic transitions of one-dimensional van Hove singularities [
2]. The energy level of the second excited state (E
2 state) is higher than that of the first excited state (E
1 state). Hence, hot electron extraction directly from the E
2 state is effective to improve the performance of photovoltaics and photocatalysts based on SWCNT-light absorbers. However, regarding a SWCNT/C
60 heterojunction, which is often used in organic solar cells, the relaxation from the E
2 state to E
1 or ground states suppresses the hot electron extraction from SWCNT to C
60 [
3]. As a result, the internal quantum efficiency (IQE) of SWCNT/C
60 solar cells depends on an energetic offset between the lowest molecular orbital (LUMO: C
1), corresponding to the E
1 state, of the nanotube and that of C
60 [
4]. In other words, the IQE of SWCNT/C
60 solar cells is not affected by the energy level of the E
2 state (LUMO+1: C
2) of SWCNTs, even upon E
22 photoexcitation with visible light. Recently, we developed SWCNT/C
60 photocatalysts that act as hydrogen evolution photocatalysts [
5,
6,
7,
8,
9]. As in the case of SWCNT/C
60 solar cells, the external quantum yield (EQY) of the photocatalytic hydrogen production increased in the order (7,5)SWCNT (0.17%) < (6,5)SWCNT (0.35%) < (8,3)SWCNT (1.5%) with increasing LUMO energy levels of the SWCNTs, despite photoexcitation using E
22 absorption to generate the E
2 state [
8]. Density functional theory (DFT) calculation has shown that E
22 excitation does not induce electron injection to C
60 in the (6,5)SWCNT/C
60 interface, although, with E
11 excitation, ultrafast electron transfer (τ < 200 fs) takes place from (6,5)SWCNT to C
60 [
10]. These observations indicate that C
60 is not capable of extracting hot electrons from SWCNTs. On the other hand, Parkinson and co-workers fabricated SWCNT heterojunctions with atomically flat surfaces of TiO
2 and SnO
2, where higher-energy second excitonic SWCNT transitions produce more photocurrent [
11]. Because of the continuum of states within the metal-oxide conduction band with a density that increases with increasing energy above the conduction band minimum, rates of carrier injection from E
2 of SWCNT to TiO
2 or SnO
2 are competitive with fast hot-exciton relaxation processes. In this context, the construction of similar photocatalytic systems is of interest in order to make photocatalytic reactions using SWCNTs more efficient. In this paper, we synthesized SWCNT/TiO
2 nanohybrids to demonstrate their photocatalytic activity for hydrogen evolution from water through the hot electron extraction from the E
2 state of SWCNT to TiO
2.
3. Results and Discussion
Water-dispersible SWCNT/BDD-dendrimer nanohybrids were synthesized by the physical modification of SWCNTs with poly(amidoamine) dendrimer with a 1,10-bis(decyloxy)decane core and carboxy (–COOH) terminals, BDD-dendrimer(COOH) (
Figure 1a) [
12,
13]. Pt-loaded TiO
2 mesocrystals (TiO
2/Pt) (25 mg) were prepared by a conventional photochemical deposition method [
12]. The Pt loading on TiO
2 was confirmed by the hydrogen production activity (0.71 μmol/h·mg) under UV irradiation. To a water dispersion (10 mL) of TiO
2/Pt (10 mg), a solution of SWCNT/BDD-dendrimer(COOH) nanohybrids (125 μL, SWCNT content 0.025 mg) was added. The mixture was stirred for 30 min and immersed overnight in the dark. The solvent was removed by decantation to obtain SWCNT/TiO
2/Pt (
Figure 1b). The amount of SWCNTs adsorbed on the TiO
2 surface was estimated to be 21 µg per 10 mg of TiO
2/Pt using the absorption spectrum of supernatant solution after the hybridization of SWCNTs with TiO
2 (
Figure S2).
Figure 2 shows SEM images of TiO
2 and SWCNTs/TiO
2/Pt. The TiO
2 mesocrystals were plate-like structures with a particle size of 10 μm (
Figure 2a), as previously reported [
12]. The plate-like structure was retained after the attachment of SWCNT/BDD-dendrimer(COOH) nanohybrids to TiO
2 (
Figure 2b). TiO
2 particles on the plate mesocrystals were stripped off by ultrasonic treatment during the Pt loading or SWCNTs attachment process. Although the amount of Pt and SWCNTs on the surface of the TiO
2 crystals was so small that they could not be observed by energy dispersive X-ray spectroscopy (EDX), HR-SEM images show the Pt nanoparticles and nanofibers on the TiO
2 mesocrystals (
Figure S3). The SWCNTs/TiO
2/Pt exhibited a Brunauer–Emmett–Teller (BET) surface area of 54.7 m
2 g
−1.
The formation of SWCNT/TiO
2/Pt nanohybrids was confirmed by absorption, 2D excitation/emission, and Raman spectra. The absorption spectra of SWCNT/TiO
2/Pt (blue line) exhibit the characteristic absorption bands derived from SWCNTs that appeared at 400–700 nm (
Figure 3). The absorption originating from (6,5)SWCNT (λ
max 570 nm) along with a small shoulder at around 670 nm originating from (8,3) and (7,5)SWCNTs were observed, almost the same as that of SWCNT/BDD-dendrimer(COOH) (orange line). Two-dimensional excitation/emission spectra show the quenching of the fluorescence of SWCNTs after the hybridization with TiO
2/Pt due to photoinduced electron transfer from SWCNTs to TiO
2 (
Figure S4).
Figure 4 shows the Raman spectrum of the SWCNT/TiO
2/Pt hybrids. Raman shifts for the G band (1585 cm
−1), D band (1316 cm
−1), and G’ band (2622 cm
−1) of the SWCNTs were observed, where the G/D ratio (3.30) did not change before or after the attachment of SWCNT onto TiO
2, indicating that the sp
2 carbon of SWCNTs was not changed to sp
3 carbon. Peaks originating from the anatase crystal of TiO
2 were observed at 403 cm
−1 (B
1g), 517 cm
−1 (A
1g), 156 cm
−1, and 643 cm
−1 (E
g) [
15]. These observations indicate that SWCNTs are adsorbed on the surface of TiO
2.
Parkinson and co-workers reported that hot electron injection from the SWCNT E
22 state to TiO
2 is more efficient than electron injection from the relaxed SWCNT E
11 state at the SWCNT/TiO
2 heterojunction due to a higher density of states (DOS) at E
22 than at E
11 of SWCNTs and the continuum of states within the TiO
2 conduction band with a density that increases with increasing energy above the conduction band edge [
11]. In marked contrast with SWCNT/C
60 heterojunctions [
3,
10], hot electron extraction from the SWCNT E
22 state (C
2) is fast enough to compete with relaxation to the E
11 state (C
1) so that the relative photon conversion efficiency (RPCE) upon E
22 photoexcitation is higher than that upon E
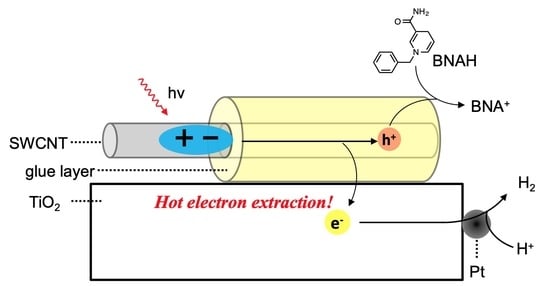
11 photoexcitation. In this context, we expected photocatalytic hydrogen evolution from water using SWCNT/TiO
2/Pt nanohybrids via direct electron extraction from SWCNT E
22 states (C
2), of which the energy level diagram is shown in
Figure 5. Higher-energy second excitonic SWCNT transition under visible-light irradiation leads to hot electron extraction from the SWCNT E
22 state (C
2) to the TiO
2 conduction band, followed by the electron migration to the Pt co-catalyst to induce the hydrogen evolution reaction. The remaining hole in the SWCNT valence bands (V
2) is consumed by simultaneous hole migration to a sacrificial donor molecule, 1-benzyl-1,4-dihydronicotinamide (BNAH). The efficiency of the hydrogen evolution reaction is dominated by the efficiency of the hot electron extraction from SWCNT E
22 to TiO
2, because the electron injection from the SWCNT E
11 state (C
1) to TiO
2 is relatively slow due to the small driving forces, although the hot-electron injection rate from C
2 to TiO
2 is competitive with hot-exciton relaxation processes, as described by Parkinson et al. [
11].
Figure 6 shows the time course of the photocatalytic hydrogen evolution reaction over SWCNT/TiO
2/Pt under visible-light irradiation (λ > 422 nm). The hydrogen production rate of 9.5 μmol/h was observed (
Figure 6, ●). The hydrogen evolution reaction continued until all of the sacrificial agent, BNAH, was consumed, and there was no induction period (
Figure S5). In contrast, no production of hydrogen was detected using TiO
2/Pt without SWCNTs under the same conditions (
Figure 6, ■), indicating that the SWCNTs act as photosensitizers and the hydrogen production reaction proceeds via electron extraction from SWCNTs on the surface of TiO
2. To compare the electron-extracting ability of TiO
2, commercially available P25 was used to synthesize SWCNT/TiO
2(P25)/Pt. Under the same reaction conditions, the hydrogen production rate of SWCNT/TiO
2(P25)/Pt was 7.3 µmol/h (
Figure S6), which is less active than that of SWCNT/TiO
2(mesocrystal)/Pt (9.5 µmol/h). The higher activity with TiO
2 mesocrystals may be due to the suppression of charge recombination at the SWCNT/TiO
2 interface. A similar result using black phosphorous/TiO
2 (mesocrystal) interface was described by Fujitsuka and co-workers [
16].
To obtain insight into this free-carrier generation process in the SWCNT/TiO
2 heterojunction, we compared the chirality dependence of EQY of the hydrogen evolution reaction using the SWCNT/TiO
2 heterojunction with that using SWCNT/C
60 upon E
22 photoexcitation of SWCNTs. In our previous reports [
8,
9], we found a commensurate reduction of EQY in the offset of the energy levels (driving force) between SWCNT C
1 and C
60 LUMO (
Figure 7). (8,3)SWCNT shows the highest EQY among (6,5), (7,5), and (8,3)SWCNTs because of the electron transfer from SWCNT to C
60 after the relaxation from the SWCNT E
22 state to the SWCNT E
11 state, as in the case of SWCNT/C
60 solar cells. If the hot electron extraction from the SWCNT E
22 state to C
60 had occurred, the EQY would depend on the energy levels of SWCNT C
2, i.e., (6,5)SWCNT would represent the highest EQY, and (8,3)SWCNT would show the lowest EQY.
In this context, we investigated the photocatalytic activity of SWCNT/TiO
2/Pt upon chirality-selective photoexcitation using monochromatic light irradiation at 570, 650, and 680 nm, which are the E
22 absorptions of (6,5), (7,5), and (8,3)SWCNTs, respectively. In a typical experiment, 150 mL of an aqueous dispersion of SWCNT/TiO
2/Pt (10 mg) and 1-benzyl-4-dihydronicotinamide (BNAH; 38.6 mg, 180 μmol/h) was exposed to monochromatic light (570, 650, or 680 nm) using a 300 W Xenon arc lamp with bandpass filters while being stirred vigorously at 25 °C. After the designated period, the gas phase above the solution was analyzed by gas chromatography.
Figure 8a (●) shows plots of the total amount of H
2 produced versus time using monochromatic light irradiation at 570 nm. A steady generation of H
2 (2.2 μmol/h) was observed without an induction period or a decrease in activity during 3 h of irradiation. Compared with the H
2 generated by the use of monochromatic light irradiation at 650 or 680 nm, 1.7 µmol/h (
Figure 8a (■)) and 1.3 µmol/h (
Figure 8a (◆)), respectively, the amount of H
2 evolution under 570 nm irradiation was the highest (2.2 μmol/h,
Figure 8a (●)). The EQYs were in the same order as for the hydrogen production rate: 5.5% for (6,5)SWCNT > 3.6% for (7,5)SWCNT > 2.2% for (8,3)SWCNT. Notably, this order of EQYs is consistent with the energy levels of the second excitonic state (C
2) for (6,5), (7,5), and (8,3)SWCNTs, –3.30, –3.54, and –3.62 eV, respectively (
Figure 9), and is the same as previous reports on the relative photon conversion efficiency (RPCE) of the SWCNT/TiO
2 heterojunction. This result indicated that the hot electron injection from the second excitonic state of SWCNTs to TiO
2 leads to a hydrogen evolution reaction in marked contrast to CNT photocatalyst based on the SWCNT/C
60 heterojunction, where the electron extraction from SWCNT to C
60 occurred after the inter-band transition from the E
22 state (C
2) to the E
11 state (C
1). Furthermore, the SWCNT/TiO
2/Pt photocatalyst exhibited higher EQYs than the previously reported SWCNT/C
60/Pt(II) photocatalyst. For example, upon 570 nm photoirradiation (E
22 absorption of (6,5)SWCNT), the EQY of SWCNT/TiO
2/Pt, 5.5%, is 16 times higher than that of SWCNT/C
60/Pt(II), 0.35%.