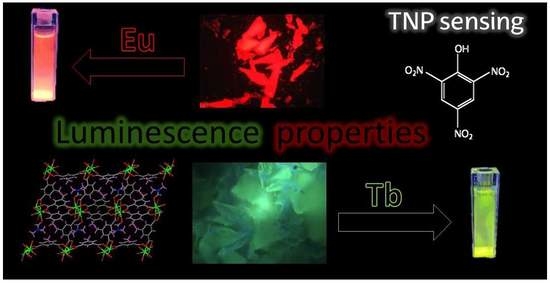
Lanthanide(III) Ions and 5-Methylisophthalate Ligand Based Coordination Polymers: An Insight into Their Photoluminescence Emission and Chemosensing for Nitroaromatic Molecules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Syntheses of [Ln2(μ4-5Meip)3(DMF)]n [Where Ln(III) = Sm (1Sm), Eu (2Eu), Gd (3Gd), Tb (4Tb) and Yb (5Yb)]
2.2. Physical Measurements
2.3. X-ray Diffraction Data Collection and Structure Determination
2.4. Photoluminescence Measurements
2.5. Luminescence Detection Experiments
2.6. Computational Details
3. Results and Discussion
3.1. Structural Description of [Ln2(μ4-5Meip)3(DMF)]n [Where Ln(III) = Sm (1Sm), Eu (2Eu), Gd (3Gd), Tb (4Tb) and Yb (5Yb)]
3.2. Luminescence Properties
3.2.1. Photoluminescence of 3Gd and H2-5Meip-Centred Emission Analysis
3.2.2. Photoluminescence Performance of Visible Emitters (1Sm, 2Eu and 4Tb)
3.2.3. Theoretical Analysis of the Luminescence on Compound 2Eu
3.2.4. Photoluminescence Performance of the NIR Emitter 5Yb
3.3. Sensing Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guillerm, V.; Kim, D.; Eubank, J.F.; Luebke, R.; Liu, X.; Adil, K.; Lah, M.S.; Eddaoudi, M. A supermolecular building approach for the design and construction of metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 6141–6172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eddaoudi, M.; Sava, D.F.; Eubank, J.F.; Adil, K.; Guillerm, V. Zeolite-like metal–organic frameworks (ZMOFs): Design, synthesis, and properties. Chem. Soc. Rev. 2015, 44, 228–249. [Google Scholar] [CrossRef] [Green Version]
- O’Keeffe, M.; Yaghi, O.M. Deconstructing the crystal structures of metal-organic frameworks and related materials into their underlying nets. Chem. Rev. 2012, 112, 675–702. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Tranchemontagne, J.L.; O’keeffe, M.; Yaghi, O.M. Secondary building units, nets and bonding in the chemistry of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1257–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batten, S.R.; Champness, N.R.; Chen, X.M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Terminology of metal-organic frameworks and coordination polymers (IUPAC recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef] [Green Version]
- Allendorf, M.D.; Stavila, V. Crystal engineering, structure–function relationships, and the future of metal–organic frameworks. CrystEngComm 2014, 17, 229–246. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Zhou, H.L.; Lin, R.B.; Zhang, C.; Lin, J.B.; Zhang, J.P.; Chen, X.M. Geometry analysis and systematic synthesis of highly porous isoreticular frameworks with a unique topology. Nat. Commun. 2012, 3, 642. [Google Scholar] [CrossRef] [Green Version]
- Cepeda, J.; Beobide, G.; Castillo, O.; Luque, A.; Pérez-Yáñez, S. Structural diversity of coordination compounds derived from double-chelating and planar diazinedicarboxylate ligands. Coord. Chem. Rev. 2017, 352, 83–107. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Mukkamala, S.B.; Jonnalagadda, S.B. A review on contemporary Metal–Organic Framework materials. Inorg. Chim. Acta 2016, 446, 61–74. [Google Scholar] [CrossRef]
- ZelěNák, V.; Saldan, I.; Giannakoudakis, D.; Barczak, M.; Pasán, J. Factors Affecting Hydrogen Adsorption in Metal–Organic Frameworks: A Short Review. Nanomaterials 2021, 11, 1638. [Google Scholar] [CrossRef]
- Zhang, J.P.; Liao, P.Q.; Zhou, H.L.; Lin, R.B.; Chen, X.M. Single-crystal X-ray diffraction studies on structural transformations of porous coordination polymers. Chem. Soc. Rev. 2014, 43, 5789–5814. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.C.; Civalleri, B. Metal–Organic Frameworks and Hybrid Materials: From Fundamentals to Applications. CrystEngComm 2014, 17, 197–198. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal-organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Abánades Lázaro, I.; Forgan, R.S. Application of zirconium MOFs in drug delivery and biomedicine. Coord. Chem. Rev. 2019, 380, 230–259. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Chen, H.; Lv, H.; Qin, Q.P.; Fan, L.; Zhang, X. Chemorobust 4p-5p {InPb}-organic framework for efficiently catalyzing cycloaddition of CO2 with epoxides and deacetalization-Knoevenagel condensation. Mater. Today Chem. 2022, 24, 100984. [Google Scholar] [CrossRef]
- Liu, S.; Chen, H.; Zhang, X. Bifunctional {Pb10K2}-Organic Framework for High Catalytic Activity in Cycloaddition of CO2with Epoxides and Knoevenagel Condensation. ACS Catal. 2022, 12, 10373–10383. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [Green Version]
- Cepeda, J.; Pérez-Yáñez, S.; Beobide, G.; Castillo, O.; Goikolea, E.; Aguesse, F.; Garrido, L.; Luque, A.; Wright, P.A. Scandium/Alkaline Metal-Organic Frameworks: Adsorptive Properties and Ionic Conductivity. Chem. Mater. 2016, 28, 2519–2528. [Google Scholar] [CrossRef]
- Fujie, K.; Ikeda, R.; Otsubo, K.; Yamada, T.; Kitagawa, H. Lithium Ion Diffusion in a Metal-Organic Framework Mediated by an Ionic Liquid. Chem. Mater. 2015, 27, 7355–7361. [Google Scholar] [CrossRef]
- Inokuma, Y.; Yoshioka, S.; Ariyoshi, J.; Arai, T.; Hitora, Y.; Takada, K.; Matsunaga, S.; Rissanen, K.; Fujita, M. X-ray analysis on the nanogram to microgram scale using porous complexes. Nature 2013, 495, 461–466. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Lin, W. Metal-organic frameworks as a tunable platform for designing functional molecular materials. J. Am. Chem. Soc. 2013, 135, 13222–13234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Y.; Chen, S.; Zhou, Y.; Zhang, Y.; Xu, M. Recent Progress in Metal–Organic Framework (MOF) Based Luminescent Chemodosimeters. Nanomaterials 2019, 9, 974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Khurshid, A.; Sohail, M.; Qiu, W.; Cao, D.; Su, S.J. Encapsulation of Dyes in Luminescent Metal-Organic Frameworks for White Light Emitting Diodes. Nanomaterials 2021, 11, 2761. [Google Scholar] [CrossRef] [PubMed]
- San Sebastian, E.; Rodríguez-Diéguez, A.; Seco, J.M.; Cepeda, J. Coordination Polymers with Intriguing Photoluminescence Behavior: The Promising Avenue for Greatest Long-Lasting Phosphors. Eur. J. Inorg. Chem. 2018, 2018, 2155–2174. [Google Scholar] [CrossRef]
- Rocha, J.; Brites, C.D.S.; Carlos, L.D. Lanthanide Organic Framework Luminescent Thermometers. Chem.—Eur. J. 2016, 22, 14782–14795. [Google Scholar] [CrossRef]
- Leo, P.; Briones, D.; García, J.A.; Cepeda, J.; Orcajo, G.; Calleja, G.; Rodríguez-Diéguez, A.; Martínez, F. Strontium-Based MOFs Showing Dual Emission: Luminescence Thermometers and Toluene Sensors. Inorg. Chem. 2020, 59, 18432–18443. [Google Scholar] [CrossRef]
- Errulat, D.; Marin, R.; Gálico, D.A.; Harriman, K.L.M.; Pialat, A.; Gabidullin, B.; Iikawa, F.; Couto, O.D.D.; Moilanen, J.O.; Hemmer, E.; et al. A Luminescent Thermometer Exhibiting Slow Relaxation of the Magnetization: Toward Self-Monitored Building Blocks for Next-Generation Optomagnetic Devices. ACS Cent. Sci. 2019, 5, 1187–1198. [Google Scholar] [CrossRef] [Green Version]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef]
- Yi, F.Y.; Chen, D.; Wu, M.K.; Han, L.; Jiang, H.L. Chemical Sensors Based on Metal–Organic Frameworks. Chempluschem 2016, 81, 675–690. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef]
- Feng, X.; Shang, Y.; Zhang, K.; Hong, M.; Li, J.; Xu, H.; Wang, L.; Li, Z. In situ ligand-induced Ln-MOFs based on a chromophore moiety: White light emission and turn-on detection of trace antibiotics. CrystEngComm 2022, 24, 4187–4200. [Google Scholar] [CrossRef]
- Feng, X.; Shang, Y.; Zhang, H.; Liu, X.; Wang, X.; Chen, N.; Wang, L.; Li, Z. Multi-functional lanthanide-CPs based on tricarboxylphenyl terpyridyl ligand as ratiometric luminescent thermometer and highly sensitive ion sensor with turn on/off effect. Dalton Trans. 2020, 49, 4741–4750. [Google Scholar] [CrossRef] [PubMed]
- Mosca, L.; Khnayzer, R.S.; Lazorski, M.S.; Danilov, E.O.; Castellano, F.N.; Anzenbacher, P. Sensing of 2,4,6-Trinitrotoluene (TNT) and 2,4-Dinitrotoluene (2,4-DNT) in the Solid State with Photoluminescent RuII and IrIII Complexes. Chem.—Eur. J. 2015, 21, 4056–4064. [Google Scholar] [CrossRef] [PubMed]
- Layfield, R.; Murugesu, M. Lanthanides and Actinides in Molecular Magnetism; Wiley: Hoboken, NJ, USA, 2015; ISBN 978-3-527-33526-8. [Google Scholar]
- Atwood, D.A. (Ed.) The Rare Earth Elements: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Bünzli, J.C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef] [PubMed]
- Heine, J.; Müller-Buschbaum, K. Engineering metal-based luminescence in coordination polymers and metal–organic frameworks. Chem. Soc. Rev. 2013, 42, 9232–9242. [Google Scholar] [CrossRef]
- Yip, Y.W.; Wen, H.; Wong, W.T.; Tanner, P.A.; Wong, K.L. Increased antenna effect of the lanthanide complexes by control of a number of terdentate n-donor pyridine ligands. Inorg. Chem. 2012, 51, 7013–7015. [Google Scholar] [CrossRef]
- Barry, D.E.; Caffrey, D.F.; Gunnlaugsson, T. Lanthanide-directed synthesis of luminescent self-assembly supramolecular structures and mechanically bonded systems from acyclic coordinating organic ligands. Chem. Soc. Rev. 2016, 45, 3244–3274. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Gavin, T.; DeCaprio, A.; Barber, D.S. Application of the Hard and Soft, Acids and Bases (HSAB) theory to toxicant—Target interactions. Chem. Res. Toxicol. 2012, 25, 239–251. [Google Scholar] [CrossRef]
- Jiang, F.L.; Gai, Y.L.; Xiong, K.C.; Chen, L.; Bu, Y.; Li, X.J.; Hong, M.C. Visible and NIR photoluminescence properties of a series of novel lanthanide-organic coordination polymers based on hydroxyquinoline-carboxylate ligands. Inorg. Chem. 2012, 51, 13128–13137. [Google Scholar] [CrossRef]
- Bruker Apex2; Bruker AXS Inc.: Billerica, MA, USA, 2004.
- Sheldrick, G.M. SADABS 1996, Program for Empirical Adsorption Correction. Available online: https://www.scienceopen.com/ (accessed on 18 March 2022).
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. FULLPROF 2000, version 2.5d. Available online: https://www.ill.eu/sites/fullprof/ (accessed on 18 May 2022).
- Li, L.; Shen, S.; Lin, R.; Bai, Y.; Liu, H. Rapid and specific luminescence sensing of Cu(II) ions with a porphyrinic metal–organic framework. Chem. Commun. 2017, 53, 9986–9989. [Google Scholar] [CrossRef] [PubMed]
- James, J.P. Stewart MOPAC2016. Available online: http://OpenMOPAC.net (accessed on 20 July 2022).
- Cepeda, J.; Pérez-Yáñez, S.; Beobide, G.; Castillo, O.; García, J.Á.; Lanchas, M.; Luque, A. Enhancing luminescence properties of lanthanide(iii)/pyrimidine-4,6-dicarboxylato system by solvent-free approach. Dalton Trans. 2015, 44, 6972–6986. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S. Continuous Shape Measures Study of the Coordination Spheres of Actinide Complexes—Part 1: Low Coordination Numbers. Eur. J. Inorg. Chem. 2021, 2021, 3632–3647. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Wang, Z.; Mo, J.T.; Fan, Y.N.; Pan, M. A long persistent phosphorescent metal–organic framework for multi-level sensing of oxygen. J. Mater. Chem. C 2020, 8, 9916–9922. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-Based Luminescent Hybrid Materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [Green Version]
- Bünzli, J.-C.G. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. [Google Scholar] [CrossRef]
- López, I.; Lorenz, K.; Nogales, E.; Méndez, B.; Piqueras, J.; Alves, E.; García, J.A. Study of the relationship between crystal structure and luminescence in rare-earth-implanted Ga2O3 nanowires during annealing treatments. J. Mater. Sci. 2014, 49, 1279–1285. [Google Scholar] [CrossRef]
- Briones, D.; Leo, P.; Cepeda, J.; Orcajo, G.; Calleja, G.; Sanz, R.; Rodríguez-Diéguez, A.; Martínez, F. Alkaline-earth metal based MOFs with second scale long-lasting phosphor behavior. CrystEngComm 2018, 20, 4793–4803. [Google Scholar] [CrossRef]
- Nyokong, T. Effects of substituents on the photochemical and photophysical properties of main group metal phthalocyanines. Coord. Chem. Rev. 2007, 251, 1707–1722. [Google Scholar] [CrossRef]
- Solov’ev, K.N.; Borisevich, E.A. Intramolecular heavy-atom effect in the photophysics of organic molecules. Physics-Uspekhi 2005, 48, 231–253. [Google Scholar] [CrossRef]
- del Valle, J.C.; Catalán, J. Kasha’s rule: A reappraisal. Phys. Chem. Chem. Phys. 2019, 21, 10061–10069. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Muelle, A.B.; García-García, A.; García-Valdivia, A.A.; Oyarzabal, I.; Cepeda, J.; Seco, J.M.; Colacio, E.; Rodríguez-Diéguez, A.; Fernández, I. Design and synthesis of a family of 1D-lanthanide-coordination polymers showing luminescence and slow relaxation of the magnetization. Dalton Trans. 2018, 47, 12783–12794. [Google Scholar] [CrossRef]
- Zabala-Lekuona, A.; Cepeda, J.; Oyarzabal, I.; Rodríguez-Diéguez, A.; García, J.A.; Seco, J.M.; Colacio, E. Rational design of triple-bridged dinuclear ZnIILnIII-based complexes: A structural, magnetic and luminescence study. CrystEngComm 2017, 19, 256–264. [Google Scholar] [CrossRef]
- Pajuelo-Corral, O.; García, J.A.; Castillo, O.; Luque, A.; Rodríguez-Diéguez, A.; Cepeda, J. Single-ion magnet and photoluminescence properties of lanthanide(Iii) coordination polymers based on pyrimidine-4,6-dicarboxylate. Magnetochemistry 2021, 7, 8. [Google Scholar] [CrossRef]
- Bigwood, R.; Gruebele, M.; Leitner, D.M.; Wolynes, P.G. The vibrational energy flow transition in organic molecules: Theory meets experiment. Proc. Natl. Acad. Sci. USA 1998, 95, 5960–5964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, M.; Ohmagari, H.; Tanaka, H.; Machida, K. Luminescence of lanthanide complexes: From fundamental to prospective approaches related to water- and molecular-stimuli. J. Photochem. Photobiol. C Photochem. Rev. 2022, 50, 100484. [Google Scholar] [CrossRef]
- Yamamoto, H.; Shionoya, S.; Yen, W.M. Phosphor Handbook, 2nd ed.; Yamamoto, H., Shionoya, S., Yen, W.M., Eds.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780849335648. [Google Scholar]
- Latva, M.; Takalob, H.; Mukkala, V.M.; Matachescu, C.; Rodríguez-Ubis, J.C.; Kankare, J. Correlation between the lowest triplet state energy level of the ligand and lanthanide(III) luminescence quantum yield. J. Lumin. 1997, 75, 149–169. [Google Scholar] [CrossRef]
- Dutra, J.D.L.; Bispo, T.D.; Freire, R.O. LUMPAC lanthanide luminescence software: Efficient and user friendly. J. Comput. Chem. 2014, 35, 772–775. [Google Scholar] [CrossRef]
- Borges, A.S.; Caliman, E.V.; Dutra, J.D.L.; Da Silva, J.G.; Araujo, M.H. Structure and luminescent investigation of new Ln(III)-TTA complexes containing N-methyl-ε-caprolactam as ligand. J. Lumin. 2016, 170, 654–662. [Google Scholar] [CrossRef]
- Malta, O.L.; Gonçalves e Silva, F.R. A theoretical approach to intramolecular energy transfer and emission quantum yields in coordination compounds of rare earth ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1998, 54, 1593–1599. [Google Scholar] [CrossRef]
- Malta, O.L. Ligand—Rare-earth ion energy transfer in coordination compounds. A theoretical approach. J. Lumin. 1997, 71, 229–236. [Google Scholar] [CrossRef]
- de Sá, G.F.; Malta, O.L.; de Mello Donegá, C.; Simas, A.M.; Longo, R.L.; Santa-Cruz, P.A.; da Silva, E.F. Spectroscopic properties and design of highly luminescent lanthanide coordination complexes. Coord. Chem. Rev. 2000, 196, 165–195. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef] [PubMed]
- Monguzzi, A.; Milani, A.; Lodi, L.; Trioni, M.I.; Tubino, R.; Castiglioni, C. Vibrational overtones quenching of near infrared emission in Er3+ complexes. New J. Chem. 2009, 33, 1542–1548. [Google Scholar] [CrossRef]
- Ermolaev, V.L.; Sveshnikova, E.B. The application of luminescence-kinetic methods in the study of the formation of lanthanide ion complexes in solution. Russ. Chem. Rev. 1994, 63, 905–922. [Google Scholar] [CrossRef]
- Cavalli, E.; Ruggieri, S.; Mizzoni, S.; Nardon, C.; Bettinelli, M.; Piccinelli, F. NIR-emission from Yb(III)- and Nd(III)-based complexes in the solid state sensitized by a ligand system absorbing in a broad UV and visible spectral window. Results Chem. 2022, 4, 100388. [Google Scholar] [CrossRef]
- Kasprzycka, E.; Carneiro Neto, A.N.; Trush, V.A.; Malta, O.L.; Jerzykiewicz, L.; Amirkhanov, V.M.; Legendziewicz, J.; Gawryszewska, P. Spectroscopic aspects for the Yb3+ coordination compound with a large energy gap between the ligand and Yb3+ excited states. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 274, 121072. [Google Scholar] [CrossRef]
- Huang, Y.L.; Qiu, P.L.; Bai, J.P.; Luo, D.; Lu, W.; Li, D. Exclusive Recognition of Acetone in a Luminescent BioMOF through Multiple Hydrogen-Bonding Interactions. Inorg. Chem. 2019, 58, 7667–7671. [Google Scholar] [CrossRef]
- Bayliss, N.S.; Mcrae, E.G. Solvent effects in the spectra of acetone, crotonaldehyde, nitromethane and nitrobenzene. J. Phys. Chem. 1954, 58, 1006–1011. [Google Scholar] [CrossRef]
- Klongdee, F.; Youngme, S.; Boonmak, J. A luminescent sensor based on zinc(II) 1D chain coordination polymer for effective acetone detection. Polyhedron 2020, 180, 114437. [Google Scholar] [CrossRef]
- Terzyk, A.P.; Bieniek, A.; Bolibok, P.; Wiśniewski, M.; Ferrer, P.; da Silva, I.; Kowalczyk, P. Stability of coordination polymers in water: State of the art and towards a methodology for nonporous materials. Adsorption 2019, 25, 1–11. [Google Scholar] [CrossRef] [Green Version]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology. IUPAC Recommendations, (the “Gold Book”), 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1997; ISBN 0-9678550-9-8. [Google Scholar]
- Nath, S.; Pathak, S.K.; Pradhan, B.; Gupta, R.K.; Reddy, K.A.; Krishnamoorthy, G.; Achalkumar, A.S. A sensitive and selective sensor for picric acid detection with a fluorescence switching response. New J. Chem. 2018, 42, 5382–5394. [Google Scholar] [CrossRef]
- Qian, L.L.; Wang, Z.X.; Ding, J.G.; Tian, H.X.; Li, K.; Li, B.L.; Li, H.Y. A 2D copper(I) metal-organic framework: Synthesis, structure and luminescence sensing for cupric, ferric, chromate and TNP. Dye. Pigment. 2020, 175, 108159. [Google Scholar] [CrossRef]
- Honglae, S.; Sailor, M.J.; Magde, D.; Trogler, W.C. Detection of Nitroaromatic Explosives Based on Photoluminescent Polymers Containing Metalloles. J. Am. Chem. Soc. 2003, 125, 3821–3830. [Google Scholar] [CrossRef]
- Zhou, X.H.; Li, L.; Li, H.H.; Li, A.; Yang, T.; Huang, W. A flexible Eu(III)-based metal–organic framework: Turn-off luminescent sensor for the detection of Fe(III) and picric acid. Dalton Trans. 2013, 42, 12403–12409. [Google Scholar] [CrossRef]
- Xu, H.; Hu, H.C.; Cao, C.S.; Zhao, B. Lanthanide Organic Framework as a Regenerable Luminescent Probe for Fe3+. Inorg. Chem. 2015, 54, 4585–4587. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, B.; Su, Z.; Hong, G.; Li, X.; Liu, Y.; Pan, Q.; Sun, J. Lanthanide-MOFs as multifunctional luminescent sensors. Inorg. Chem. Front. 2022, 9, 3259–3266. [Google Scholar] [CrossRef]
- Liu, W.; Huang, X.; Xu, C.; Chen, C.; Yang, L.; Dou, W.; Chen, W.; Yang, H.; Liu, W. A Multi-responsive Regenerable Europium–Organic Framework Luminescent Sensor for Fe3+, CrVI Anions, and Picric Acid. Chem.-Eur. J. 2016, 22, 18769–18776. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Chu, T.; Wang, Z.; Li, W.; Yang, Y. A facile fabrication of electrodeposited luminescent MOF thin films for selective and recyclable sensing of nitroaromatic explosives. Analyst 2016, 141, 4502–4510. [Google Scholar] [CrossRef]
- He, H.; Chen, S.H.; Zhang, D.Y.; Yang, E.C.; Zhao, X.J. A luminescent metal–organic framework as an ideal chemosensor for nitroaromatic compounds. RSC Adv. 2017, 7, 38871–38876. [Google Scholar] [CrossRef] [Green Version]
- Song, X.Z.; Song, S.Y.; Zhao, S.N.; Hao, Z.M.; Zhu, M.; Meng, X.; Wu, L.L.; Zhang, H.J. Single-Crystal-to-Single-Crystal Transformation of a Europium(III) Metal–Organic Framework Producing a Multi-responsive Luminescent Sensor. Adv. Funct. Mater. 2014, 24, 4034–4041. [Google Scholar] [CrossRef]
- Cao, L.H.; Shi, F.; Zhang, W.M.; Zang, S.Q.; Mak, T.C.W. Selective Sensing of Fe3+ and Al3+ Ions and Detection of 2,4,6-Trinitrophenol by a Water-Stable Terbium-Based Metal–Organic Framework. Chem. Eur. J. 2015, 21, 15705–15712. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, J.V.; McGuffin, V.L. Rapid and accurate determination of Stern-Volmer quenching constants. Appl. Spectrosc. 1999, 53, 1000–1008. [Google Scholar]
- Kusba, J.; Bogdanov, V.; Gryczynski, I.; Lakowicz, J.R. Theory of light quenching: Effects of fluorescence polarization, intensity, and anisotropy decays. Biophys. J. 1994, 67, 2024–2040. [Google Scholar] [CrossRef]








| Compound | 1Sm | 2Eu |
|---|---|---|
| Empirical formula | C30H25Sm2NO13 | C30H25Eu2NO13 |
| Formula weight | 908.21 | 911.43 |
| Crystal system | monoclinic | monoclinic |
| Space group | P21/c | P21/c |
| a (Å) | 19.662(1) | 19.618(1) |
| b (Å) | 8.3368(6) | 8.3105(7) |
| c (Å) | 18.794(1) | 18.764(1) |
| β (°) | 104.867(3) | 104.959(3) |
| V (Å3) | 2977.5(4) | 2955.4(5) |
| Reflections collected | 26,186 | 31,478 |
| Unique data/parameters | 6814/420 | 5215/420 |
| Rint | 0.0285 | 0.0424 |
| GoF (S) 1 | 1.081 | 1.095 |
| R1 2/wR2 [I > 2σ(I)] 3 | 0.0521/0.1343 | 0.0288/0.0642 |
| R1 2/wR2 [all] 3 | 0.0608/0.1419 | 0.0343/0.0668 |
| Coordination sphere of the Eu1 atom | |||
| Eu1–O1A | 2.353(4) | Eu1–O2B(iv) | 2.335(4) |
| Eu1–O1B | 2.300(4) | Eu1-O3B(i) | 2.656(3) |
| Eu1–O1D | 2.476(4) | Eu1-O3B(v) | 2.436(3) |
| Eu1–O2A(iii) | 2.403(4) | Eu1–O4B(i) | 2.413(4) |
| Coordination sphere of the Eu2 atom | |||
| Eu2–O1C | 2.315(4) | Eu2–O4A | 2.426(3) |
| Eu2–O2C | 2.296(3) | Eu2–O4A(vi) | 2.626(3) |
| Eu2–O3A(iv) | 2.429(4) | Eu2–O4C(v) | 2.306(4) |
| Eu2–O3C(vii) | 2.342(4) | ||
| MOF | Medium | Ksv (M−1) | Ref. |
|---|---|---|---|
| {[Eu2(L1)1.5(H2O)2EtOH]·DMF}n | DMF | 2.0 × 103 | [87] |
| {[Eu2(TDC)3(CH3OH)2]·CH3OH}n | Methanol | 1.1 × 104 | [88] |
| [Pr2(TATMA)2·4DMF·4H2O]n | DMF | 1.6 × 104 | [89] |
| {[Eu3(bpydb)3(HCOO)(µ3-OH)2(DMF)]·3DMF·H2O}n | Water | 2.1 × 104 | [90] |
| {[Tb(L2)1.5(H2O)]·3H2O}n | Water | 7.5 × 104 | [91] |
| [Eu(BDPO)(H2O)4]n | Water | 6.0 × 105 | [90] |
| [Tb(BDPO)(H2O)4]n | Water | 6.0 × 105 | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajuelo-Corral, O.; Razquin-Bobillo, L.; Rojas, S.; García, J.A.; Choquesillo-Lazarte, D.; Salinas-Castillo, A.; Hernández, R.; Rodríguez-Diéguez, A.; Cepeda, J. Lanthanide(III) Ions and 5-Methylisophthalate Ligand Based Coordination Polymers: An Insight into Their Photoluminescence Emission and Chemosensing for Nitroaromatic Molecules. Nanomaterials 2022, 12, 3977. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12223977
Pajuelo-Corral O, Razquin-Bobillo L, Rojas S, García JA, Choquesillo-Lazarte D, Salinas-Castillo A, Hernández R, Rodríguez-Diéguez A, Cepeda J. Lanthanide(III) Ions and 5-Methylisophthalate Ligand Based Coordination Polymers: An Insight into Their Photoluminescence Emission and Chemosensing for Nitroaromatic Molecules. Nanomaterials. 2022; 12(22):3977. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12223977
Chicago/Turabian StylePajuelo-Corral, Oier, Laura Razquin-Bobillo, Sara Rojas, Jose Angel García, Duane Choquesillo-Lazarte, Alfonso Salinas-Castillo, Ricardo Hernández, Antonio Rodríguez-Diéguez, and Javier Cepeda. 2022. "Lanthanide(III) Ions and 5-Methylisophthalate Ligand Based Coordination Polymers: An Insight into Their Photoluminescence Emission and Chemosensing for Nitroaromatic Molecules" Nanomaterials 12, no. 22: 3977. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12223977