Polypyrrole-Assisted Ag Doping Strategy to Boost Co(OH)2 Nanosheets on Ni Foam as a Novel Electrode for High-Performance Hybrid Supercapacitors
Abstract
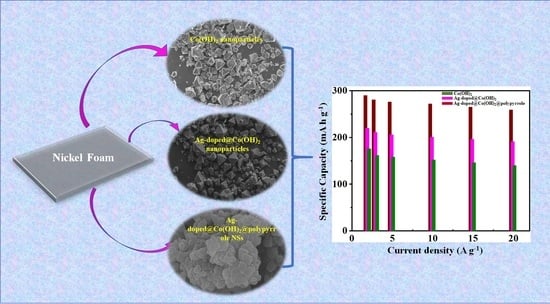
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Ag-Doped@Co(OH)2 Nanoparticles
2.3. Preparation of Ag-Doped@Co(OH)2@Polypyrrole Nanosheets
2.4. Measurements and Characterizations
3. Results and Discussion
Electrochemical Properties of Electrode Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, Y.; Mathis, T.S.; Zhao, M.Q.; Anasori, B.; Dang, A.; Zhou, Z.; Cho, H.; Gogotsi, Y.; Yang, S. Thickness-independent capacitance of vertically aligned liquid-crystalline MXenes. Nature 2018, 557, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.; Sun, Y.; Liu, Y.; Xia, Y.; Liu, X.; Gao, Y.; Wu, S.; Li, T.; Zada, A.; Ye, F. Flexible Ti3C2Tx/Carbon Nanotubes/CuS Film Electrodes Based on a Dual-Structural Design for High-Performance All-Solid-State Supercapacitors. ACS Appl. Energy Mater. 2022, 5, 9158–9172. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Shao, Y.; Kaner, R.B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 16033. [Google Scholar] [CrossRef]
- Dang, A.; Sun, Y.; Fang, C.; Li, T.; Liu, X.; Xia, Y.; Ye, F.; Zada, A.; Khan, M. Rational design of Ti3C2/carbon nanotubes/MnCo2S4 electrodes for symmetric supercapacitors with high energy storage. Appl. Surf. Sci. 2022, 581, 152432. [Google Scholar] [CrossRef]
- Kumar, K.S.; Choudhary, N.; Jung, Y.; Thomas, J. Recent Advances in Two-Dimensional Nanomaterials for Supercapacitor Electrode Applications. ACS Energy Lett. 2018, 3, 482–495. [Google Scholar] [CrossRef]
- Dang, A.; Li, T.; Xiong, C.; Zhao, T.; Shang, Y.; Liu, H.; Chen, X.; Li, H.; Zhuang, Q.; Zhang, S. Long-life electrochemical supercapacitor based on a novel hierarchically carbon foam templated carbon nanotube electrode. Compos. Part B Eng. 2018, 141, 250–257. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kumar, Y.A.; Sambasivam, S.; Hira, S.A.; Krishna, T.N.V.; Zeb, K.; Uddin, W.; Kumar, K.D.; Obaidat, I.M.; Kim, S.; et al. Highly efficient copper-cobalt sulfide nano-reeds array with simplistic fabrication strategy for battery-type supercapacitors. J. Energy Storage 2020, 32, 101988. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, T.; Zhang, Y.; Li, B.; Han, Q.; Li, D.; Ni, Y. Li–Na metal compounds inserted into porous natural wood as a bifunctional hybrid applied in supercapacitors and electrocatalysis. Int. J. Hydrogen Energy 2022, 47, 2389–2398. [Google Scholar] [CrossRef]
- Ning, W.W.; Chen, L.B.; Wei, W.F.; Chen, Y.J.; Zhang, X.Y. NiCoO2/NiCoP@Ni nanowire arrays: Tunable composition and unique structure design for high-performance winding asymmetric hybrid supercapacitors. Rare Met. 2020, 39, 1034–1044. [Google Scholar] [CrossRef]
- Xiong, C.; Zheng, C.; Li, B.; Ni, Y. Wood-based micro-spring composite elastic material with excellent electrochemical performance, high elasticity and elastic recovery rate applied in supercapacitors and sensors. Ind. Crops Prod. 2022, 178, 114565. [Google Scholar] [CrossRef]
- Anil Kumar, Y.; Sambasivam, S.; Ahmed Hira, S.; Zeb, K.; Uddin, W.; Krishna, T.N.V.; Dasha Kumar, K.; Obaidat, I.M.; Kim, H.-J. Boosting the Energy Density of Highly Efficient Flexible Hybrid Supercapacitors via Selective Integration of Hierarchical Nanostructured Energy Materials. Electrochim. Acta 2020, 364, 137318. [Google Scholar] [CrossRef]
- Muralee Gopi, C.V.V.; Vinodh, R.; Sambasivam, S.; Obaidat, I.M.; Naidu Kalla, R.M.; Kim, H.-J. One-Pot Synthesis of Copper Oxide–Cobalt Oxide Core–Shell Nanocactus-like Heterostructures as Binder-Free Electrode Materials for High-Rate Hybrid Supercapacitors. Mater. Today Energy 2019, 14, 100358. [Google Scholar] [CrossRef]
- Yedluri, A.K.; Kim, H.-J. Wearable Super-High Specific Performance Supercapacitors Using a Honeycomb with Folded Silk-like Composite of NiCo2O4 Nanoplates Decorated with NiMoO4 Honeycombs on Nickel Foam. Dalton Trans. 2018, 47, 15545–15554. [Google Scholar] [CrossRef] [PubMed]
- Yedluri, A.K.; Anitha, T.; Kim, H.-J. Fabrication of Hierarchical NiMoO4/NiMoO4 Nanoflowers on Highly Conductive Flexible Nickel Foam Substrate as a Capacitive Electrode Material for Supercapacitors with Enhanced Electrochemical Performance. Energies 2019, 12, 1143. [Google Scholar] [CrossRef] [Green Version]
- Kumar, Y.A.; Kim, H.-J. Preparation and Electrochemical Performance of NiCo2O4@NiCo2O4 Composite Nanoplates for High Performance Supercapacitor Applications. New J. Chem. 2018, 42, 19971–19978. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kumar, Y.A.; Sambasivam, S.; Hira, S.A.; Zeb, K.; Uddin, W.; Reddy, P.R.S.; Kumar, K.D.; Obaidat, I.M.; Kim, H.-J.; et al. CoCu2O4 Nanoflowers Architecture as an Electrode Material for Battery Type Supercapacitor with Improved Electrochemical Performance. Nano-Struct. Nano-Objects 2020, 24, 100618. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Kumar, K.D.; Kim, H.J. A novel electrode for supercapacitors: Efficient PVP-assisted synthesis of Ni3S2 nanostructures grown on Ni foam for energy storage. Dalton Trans. 2020, 49, 4050–4059. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, Z.-S.; Zhou, F.; Shi, X.; Zheng, S.; Qin, J.; Xiao, H.; Sun, C.; Bao, X. All-Solid-State High-Energy Planar Hybrid Micro-Supercapacitors Based on 2D VN Nanosheets and Co(OH)2 Nanoflowers. Npj Mater. Appl. 2018, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Gui, Q.; Jiang, J.; Li, Y.; Liu, J. One-Pot Growth of Co(OH)2 Nanowire Bundle Arrays on in Situ Functionalized Carbon Cloth for Robust Flexible Supercapacitor Electrodes. Dalton Trans. 2018, 47, 15416–15423. [Google Scholar] [CrossRef]
- Farhadi, S.; Pourzare, K.; Sadeghinejad, S. Simple Preparation of Ferromagnetic Co3O4 Nanoparticles by Thermal Dissociation of the [CoII(NH3)6](NO3)2 Complex at Low Temperature. J. Nanostruct. Chem. 2013, 3, 16. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, G.; Ge, B.; Zhou, H.; Yuan, A.; Shen, X. Concave Co3O4 Octahedral Mesocrystal: Polymer-Mediated Synthesis and Sensing Properties. CrystEngComm 2012, 14, 6264–6270. [Google Scholar] [CrossRef]
- Mariammal, R.N.; Ramachandran, K.; Kalaiselvan, G.; Arumugam, S.; Renganathan, B.; Sastikumar, D. Effect of Magnetism on the Ethanol Sensitivity of Undoped and Mn-Doped CuO Nanoflakes. Appl. Surf. Sci. 2013, 270, 545–552. [Google Scholar] [CrossRef]
- Aghazadeh, M. Electrochemical Preparation and Properties of Nanostructured Co3O4 as Supercapacitor Material. J. Appl. Electrochem. 2012, 42, 89–94. [Google Scholar] [CrossRef]
- Khalil, T.E.; Soliman, S.M.; Khalil, N.A.; El-Faham, A.; Foro, S.; El-Dissouky, A. Synthesis, Structure, X-ray Photoelectron Spectroscopy (XPS), and Antimicrobial, Anticancer, and Antioxidant Activities of Co (III) Complexes Based on the Antihypertensive Hydralazine. Appl. Organomet. Chem. 2022, 36, e6565. [Google Scholar] [CrossRef]
- Aadil, M.; Zulfiqar, S.; Shahid, M.; Haider, S.; Shakir, I.; Warsi, M.F. Binder free mesoporous Ag-doped Co3O4 nanosheets with outstanding cyclic stability and rate capability for advanced supercapacitor applications. J. Alloys Compd. 2020, 844, 156062. [Google Scholar] [CrossRef]
- Kulurumotlakatla, D.K.; Yedluri, A.K.; Kim, H.J. Hierarchical NiCo2S4 nanostructure as highly efficient electrode material for high-performance supercapacitor applications. J. Energy Storage 2020, 31, 101619. [Google Scholar] [CrossRef]
- Mazinani, B.; Kazazi, M.; Mobarhan, G.; Shokouhimehr, M. The Combustion Synthesis of Ag-Doped MnCo2O4 Nanoparticles for Supercapacitor Applications. JOM 2019, 71, 1499–1506. [Google Scholar] [CrossRef]
- Wang, Y.; Zhitomirsky, I. Cathodic electrodeposition of Ag-doped manganese dioxide films for electrodes of electrochemical supercapacitors. Mater. Lett. 2011, 65, 1759–1761. [Google Scholar] [CrossRef]
- Mahieddine, A.; Adnane-Amara, L.; Gabouze, N.; Addad, A.; Swaidan, A.; Boukherroub, R. Self-Combustion Synthesis of Dilithium Cobalt Bis(Tungstate) Decorated with Silver Nanoparticles for High Performance Hybrid Supercapacitors. Chem. Eng. J. 2021, 426, 131252. [Google Scholar] [CrossRef]
- Li, X.; Khalafallah, D.; Wu, Z.; Zhi, M.; Hong, Z. Silver Incorporated Partially Reduced NiCo-Layered Double Hydroxide Frameworks for Asymmetric Supercapacitors. J. Energy Storage 2020, 31, 101578. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Deng, T.; Zhang, W. Ni(OH)2 Derived Ni-MOF Supported on Carbon Nanowalls for Supercapacitors. Nanotechnology 2021, 32, 195404. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Dai, X.; Lv, G.; Wei, X.; Li, S.; Li, Z.; Xue, T.; Shi, M.; Zou, K.; Chen, Y.; et al. Stability-Enhanced α-Ni(OH)2 Pillared by Metaborate Anions for Pseudocapacitors. ACS Appl. Mater. Interfaces 2021, 13, 28118–28128. [Google Scholar] [CrossRef]
- Zhao, Y.; Ran, W.; He, J.; Huang, Y.; Liu, Z.; Liu, W.; Tang, Y.; Zhang, L.; Gao, D.; Gao, F. High-Performance Asymmetric Supercapacitors Based on Multilayer MnO2/Graphene Oxide Nanoflakes and Hierarchical Porous Carbon with Enhanced Cycling Stability. Small 2015, 11, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Wang, X.; Li, P.; Shao, H.; Li, T.; Liu, H.; Zheng, Q.; Hu, J.; Duan, L.; et al. Electro-Synthesized Co(OH)2 @CoSe with Co–OH Active Sites for Overall Water Splitting Electrocatalysis. Nanoscale Adv. 2020, 2, 792–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, L.-F.; Li, C.-F.; Zhao, J.-W.; Xie, L.-J.; Wu, J.-Q.; Ren, Q.; Li, G.-R. Dual Modulation of Lattice Strain and Charge Polarization Induced by Co(OH)2/Ni(OH)2 Interfaces for Efficient Oxygen Evolution Catalysis. J. Mater. Chem. A 2021, 9, 13279–13287. [Google Scholar] [CrossRef]
- Wu, J.Z.; Tu, J.P.; Yuan, Y.F.; Ma, M.; Wang, X.L.; Zhang, L.; Li, R.L.; Zhang, J. Ag-Modification Improving the Electrochemical Performance of ZnO Anode for Ni/Zn Secondary Batteries. J. Alloys Compd. 2009, 479, 624–628. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D.; Long, J.W. To Be or Not To Be Pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.-W.; Yin, B.-S.; Liu, X.-X.; Gu, D.-M.; Gong, H.; Wang, Z.-B. A High Energy Density Aqueous Hybrid Supercapacitor with Widened Potential Window through Multi Approaches. Nano Energy 2019, 59, 41–49. [Google Scholar] [CrossRef]
- Hu, L.; Chen, W.; Xie, X.; Liu, N.; Yang, Y.; Wu, H.; Yao, Y.; Pasta, M.; Alshareef, H.N.; Cui, Y. Symmetrical MnO2–Carbon Nanotube–Textile Nanostructures for Wearable Pseudocapacitors with High Mass Loading. ACS Nano 2011, 5, 8904–8913. [Google Scholar] [CrossRef]
- Cao, R.; Yang, H.; Deng, X.; Sun, P.; Zhang, S.; Xu, X. Construction of 3DOM Carbon Nitrides with Quasi-Honeycomb Structures for Efficient Photocatalytic H2 Production. ChemCatChem 2018, 10, 5656–5664. [Google Scholar] [CrossRef]
- Li, H.; Ma, S.; Cai, H.; Zhou, H.; Huang, Z.; Hou, Z.; Wu, J.; Yang, W.; Yi, H.; Fu, C.; et al. Ultra-Thin Fe3C Nanosheets Promote the Adsorption and Conversion of Polysulfides in Lithium-Sulfur Batteries. Energy Storage Mater. 2019, 18, 338–348. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Jia, H.; Liu, Z.; Wang, L.; Sheng, J.; Fei, W. A Highly Conductive Ni(OH)2 Nano-Sheet Wrapped CuCo2S4 Nano-Tube Electrode with a Core–Shell Structure for High Performance Supercapacitors. Dalton Trans. 2021, 50, 8476–8486. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Lin, H.; Lu, H.; Xing, E.; Zhang, Y.; Xie, B. Synthesis of Hierarchically Porous MnO2/Rice Husks Derived Carbon Composite as High-Performance Electrode Material for Supercapacitors. Appl. Energy 2016, 178, 260–268. [Google Scholar] [CrossRef]
- Dong, B.; Li, M.; Chen, S.; Ding, D.; Wei, W.; Gao, G.; Ding, S. Formation of G-C3N4@Ni(OH)2 Honeycomb Nanostructure and Asymmetric Supercapacitor with High Energy and Power Density. ACS Appl. Mater. Interfaces 2017, 9, 17890–17896. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhong, K.; Liu, C.; Yang, Y.; Zhao, Z.; Li, T.; Lu, Q. Size-Controlled Ag Quantum Dots Decorated on Binder-Free Hierarchical NiCoP Films by Magnetron Sputtering to Boost Electrochemical Performance for Supercapacitors. Nanoscale 2021, 13, 7761–7773. [Google Scholar] [CrossRef]
- Jayababu, N.; Jo, S.; Kim, Y.; Kim, D. Novel Conductive Ag-Decorated NiFe Mixed Metal Telluride Hierarchical Nanorods for High-Performance Hybrid Supercapacitors. ACS Appl. Mater. Interfaces 2021, 13, 19938–19949. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.; Sekhar, S.C.; Ramulu, B.; Yu, J.S. An Integrated Approach Toward Renewable Energy Storage Using Rechargeable [email protected] Hybrid Supercapacitors. Small 2019, 15, 1805418. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Abas, A.; Zhang, Y.; Mu, X.; Zhou, J.; Su, Q.; Lan, W.; Xie, E. Ag Nanoparticles Enhanced Vertically-Aligned CuO Nanowire Arrays Grown on Cu Foam for Stable Hybrid Supercapacitors with High Energy Density. Electrochim. Acta 2019, 296, 535–544. [Google Scholar] [CrossRef]
- Sheng, H.; Zhang, X.; Ma, Y.; Wang, P.; Zhou, J.; Su, Q.; Lan, W.; Xie, E.; Zhang, C.J. Ultrathin, Wrinkled, Vertically Aligned Co(OH)2 Nanosheets/Ag Nanowires Hybrid Network for Flexible Transparent Supercapacitor with High Performance. ACS Appl. Mater. Interfaces 2019, 11, 8992–9001. [Google Scholar] [CrossRef]
- Noori, A.; El-Kady, M.F.; Rahmanifar, M.S.; Kaner, R.B.; Mousavi, M.F. Towards Establishing Standard Performance Metrics for Batteries, Supercapacitors and Beyond. Chem. Soc. Rev. 2019, 48, 1272–1341. [Google Scholar] [CrossRef]
- Liao, J.; Wang, X.; Wang, Y.; Su, S.; Nairan, A.; Kang, F.; Yang, C. Lavender-like Cobalt Hydroxide Nanoflakes Deposited on Nickel Nanowire Arrays for High-Performance Supercapacitors. RSC Adv. 2018, 8, 17263–17271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.; Li, G.; Zhao, L. Ni-Co-S/Co(OH)2 Nanocomposite for High Energy Density All-Solid-State Asymmetric Supercapacitors. Chem. Eng. J. 2018, 336, 602–611. [Google Scholar] [CrossRef]
- Soram, B.S.; Dai, J.; Kshetri, T.; Kim, N.H.; Lee, J.H. Vertically Grown and Intertwined Co(OH)2 Nanosheet@Ni-Mesh Network for Transparent Flexible Supercapacitor. Chem. Eng. J. 2020, 391, 123540. [Google Scholar] [CrossRef]
- Li, X.J.; Xing, W.; Zhou, J.; Wang, G.Q.; Zhuo, S.P.; Yan, Z.F.; Xue, Q.Z.; Qiao, S.Z. Excellent Capacitive Performance of a Three-Dimensional Hierarchical Porous Graphene/Carbon Composite with a Superhigh Surface Area. Chem.–A Eur. J. 2014, 20, 13314–13320. [Google Scholar] [CrossRef]
- Bao, L.; Li, T.; Chen, S.; He, Y.; Peng, C.; Li, L.; Xu, Q.; Ou, E.; Xu, W. Electronic Channel in 3D Flowery Co(OH)2/N-Doped Graphene Composites with Enhanced Electrochemistry Performance. Mater. Lett. 2016, 185, 72–76. [Google Scholar] [CrossRef]
- Cheng, J.P.; Liu, L.; Ma, K.Y.; Wang, X.; Li, Q.Q.; Wu, J.S.; Liu, F. Hybrid Nanomaterial of α-Co(OH)2 Nanosheets and Few-Layer Graphene as an Enhanced Electrode Material for Supercapacitors. J. Colloid Interface Sci. 2017, 486, 344–350. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Wang, S.; Wang, Y.; Zhao, Y.; Zheng, W. Synthesis of Co(OH)2/Graphene/Ni Foam Nano-Electrodes with Excellent Pseudocapacitive Behavior and High Cycling Stability for Supercapacitors. Int. J. Hydrogen Energy 2012, 37, 11846–11852. [Google Scholar] [CrossRef]
- Mondal, C.; Ghosh, D.; Ganguly, M.; Sasmal, A.K.; Roy, A.; Pal, T. Synthesis of Multiwall Carbon Nanotube Wrapped Co(OH)2 Flakes: A High-Performance Supercapacitor. Appl. Surf. Sci. 2015, 359, 500–507. [Google Scholar] [CrossRef]
- Li, M.; Ma, K.Y.; Cheng, J.P.; Lv, D.; Zhang, X.B. Nickel–Cobalt Hydroxide Nanoflakes Conformal Coating on Carbon Nanotubes as a Supercapacitive Material with High-Rate Capability. J. Power Sources 2015, 286, 438–444. [Google Scholar] [CrossRef]
- Pang, H.; Li, X.; Zhao, Q.; Xue, H.; Lai, W.-Y.; Hu, Z.; Huang, W. One-Pot Synthesis of Heterogeneous Co3O4-Nanocube/Co(OH)2-Nanosheet Hybrids for High-Performance Flexible Asymmetric All-Solid-State Supercapacitors. Nano Energy 2017, 35, 138–145. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Z.H.; Wang, Z.G.; Zhang, F.X.; Jin, J. Layered α-Co(OH)2 Nanocones as Electrode Materials for Pseudocapacitors: Understanding the Effect of Interlayer Space on Electrochemical Activity. Adv. Funct. Mater. 2013, 23, 2758–2764. [Google Scholar] [CrossRef]
- Wang, Z.; Hong, P.; Peng, S.; Zou, T.; Yang, Y.; Xing, X.; Wang, Z.; Zhao, R.; Yan, Z.; Wang, Y. Co(OH)2@FeCo2O4 as Electrode Material for High Performance Faradaic Supercapacitor Application. Electrochim. Acta 2019, 299, 312–319. [Google Scholar] [CrossRef]






| Electrode | Fabrication Method | Electrolyte | Capacitance (Current Density) | Cycling Stability (no. of Cycles) | Ref. |
|---|---|---|---|---|---|
| Co(OH)xCO3 with hierarchical flowery architecture | Hydrothermal | 2 M KOH | 550 F g−1 (2 Ag−1) | 99.5% (1500) | [54] |
| Flower-like Co(OH)2/N-doped graphene composite | Hydrothermal | 3 M KOH | 2276 F g−1 (1 Ag−1) | 93.5% (2000) | [55] |
| Delaminated α-Co(OH)2@graphene | Hydrothermal and calcination | 2 M KOH | 567 F g−1 (1 Ag−1) | 82% (2000) | [56] |
| Co(OH)2/graphene/Ni foam nanoelectrodes | Hydrothermal | 6 M KOH | 694 F g−1 (2 Ag−1) | 91.9% (3000) | [57] |
| CNT-wrapped Co(OH)2 flakes | Hydrothermal-Electrodeposition | 2 M KOH | 603 F g−1 (1 mV s−1) | 96% (1000) | [58] |
| Ni–Co hydroxide/CNT composites | One-step hydrothermal | 2 M KOH | 1151 F g−1 (1 Ag−1) | 77% (10000) | [59] |
| Heterogeneous Co3O4-nanocube/Co(OH)2-nanosheet hybrid | Hydrothermal-thermal annealing | 6 M KOH | 1164 F g−1 (1.2 Ag−1) | 97.4% (6000) | [60] |
| Layered α-Co(OH)2 nanocones | Hydrothermal reaction | 3 M KOH | 1055 F g−1 (1 Ag−1) | 95% (2000) | [61] |
| Co(OH)2@FeCo2O4 | Hydrothermal | 6 M KOH | 1173.43 F g−1 (1 Ag−1) | 95.4% (5000) | [62] |
| Ag-doped@Co(OH)2@polypyrrole NSs | Hydrothermal | 2 M KOH | 291.2 mAh g−1 mA h g−1 or 1734.3 F g−1 (2 A g−1) | 84% (5000) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arbi, H.M.; Yadav, A.A.; Anil Kumar, Y.; Moniruzzaman, M.; Alzahmi, S.; Obaidat, I.M. Polypyrrole-Assisted Ag Doping Strategy to Boost Co(OH)2 Nanosheets on Ni Foam as a Novel Electrode for High-Performance Hybrid Supercapacitors. Nanomaterials 2022, 12, 3982. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12223982
Arbi HM, Yadav AA, Anil Kumar Y, Moniruzzaman M, Alzahmi S, Obaidat IM. Polypyrrole-Assisted Ag Doping Strategy to Boost Co(OH)2 Nanosheets on Ni Foam as a Novel Electrode for High-Performance Hybrid Supercapacitors. Nanomaterials. 2022; 12(22):3982. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12223982
Chicago/Turabian StyleArbi, Hammad Mueen, Anuja A. Yadav, Yedluri Anil Kumar, Md Moniruzzaman, Salem Alzahmi, and Ihab M. Obaidat. 2022. "Polypyrrole-Assisted Ag Doping Strategy to Boost Co(OH)2 Nanosheets on Ni Foam as a Novel Electrode for High-Performance Hybrid Supercapacitors" Nanomaterials 12, no. 22: 3982. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12223982