A Fluorescent Nanosensor for Silver (Ag+) and Mercury (Hg2+) Ions Using Eu (III)-Doped Carbon Dots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Synthesis of Doped and Undoped Cdots
3. Results and Discussion
3.1. Structural and Morphological Characterization
3.2. Optical Properties
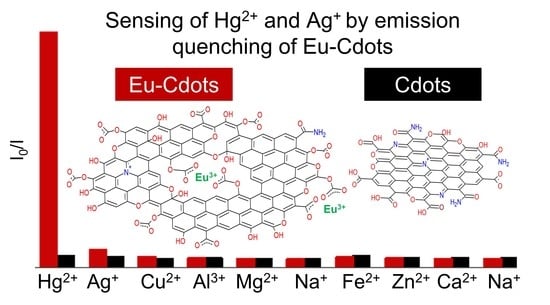
3.3. Quenching of Emission by Silver and Mercury
3.4. Mechanistic Insight
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, S.J.; Song, Y.B.; Zhao, X.H.; Shao, J.R.; Zhang, J.H.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Zhang, J.F.; Zhou, Y.; Yoon, J.; Kim, J.S. Recent progress in fluorescent and colorimetric chemosensors for detection of precious metal ions (silver, gold and platinum ions). Chem. Soc. Rev. 2011, 40, 3416–3429. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Das, P.; Bose, M.; Mondal, S.; Das, A.K.; Das, N.C. Strongly blue-luminescent N-doped carbogenic dots as a tracer metal sensing probe in aqueous medium and its potential activity towards in situ Ag-nanoparticle synthesis. Sens. Actuators B-Chem. 2017, 252, 735–746. [Google Scholar] [CrossRef]
- Das, P.; Maruthapandi, M.; Saravanan, A.; Natan, M.; Jacobi, G.; Banin, E.; Gedanken, A. Carbon Dots for Heavy-Metal Sensing, pH-Sensitive Cargo Delivery, and Antibacterial Applications. ACS Appl. Nano Mater. 2020, 3, 11777–11790. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Margel, S.; Gedanken, A. Tailor made magnetic nanolights: Fabrication to cancer theranostics applications. Nanoscale Adv. 2021, 3, 6762–6796. [Google Scholar] [CrossRef]
- Hai, X.; Feng, J.; Chen, X.W.; Wang, J.H. Tuning the optical properties of graphene quantum dots for biosensing and bioimaging. J. Mater. Chem. B 2018, 6, 3219–3234. [Google Scholar] [CrossRef]
- Lin, L.P.; Song, X.H.; Chen, Y.Y.; Rong, M.C.; Zhao, T.T.; Jiang, Y.Q.; Wang, Y.; Chen, X. One-pot synthesis of highly greenish-yellow fluorescent nitrogen-doped graphene quantum dots for pyrophosphate sensing via competitive coordination with Eu3+ ions. Nanoscale 2015, 7, 15427–15433. [Google Scholar] [CrossRef]
- Qian, Z.S.; Ma, J.J.; Shan, X.Y.; Feng, H.; Shao, L.X.; Chen, J.R. Highly Luminescent N-Doped Carbon Quantum Dots as an Effective Multifunctional Fluorescence Sensing Platform. Chem.-Eur. J. 2014, 20, 2254–2263. [Google Scholar] [CrossRef]
- Gao, X.H.; Lu, Y.Z.; Zhang, R.Z.; He, S.J.; Ju, J.; Liu, M.M.; Li, L.; Chen, W. One-pot synthesis of carbon nanodots for fluorescence turn-on detection of Ag+ based on the Ag+-induced enhancement of fluorescence. J. Mater. Chem. C 2015, 3, 2302–2309. [Google Scholar] [CrossRef]
- Cayuela, A.; Soriano, M.L.; Kennedy, S.R.; Steed, J.W.; Valcarcel, M. Fluorescent carbon quantum dot hydrogels for direct determination of silver ions. Talanta 2016, 151, 100–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.R.; Su, R.G.; Zhong, J.; Fei, L.; Cai, W.; Guan, Q.W.; Li, W.; Li, N.; Chen, Y.; Cai, L.; et al. Red/orange dual-emissive carbon dots for pH sensing and cell imaging. Nano Res. 2019, 12, 815–821. [Google Scholar] [CrossRef]
- Bian, S.Y.; Shen, C.; Qian, Y.T.; Liu, J.Y.; Xi, F.N.; Dong, X.P. Facile synthesis of sulfur-doped graphene quantum dots as fluorescent sensing probes for Ag+ ions detection. Sens. Actuators B-Chem. 2017, 242, 231–237. [Google Scholar] [CrossRef]
- Song, T.; Zhu, X.F.; Zhou, S.H.; Yang, G.; Gan, W.; Yuan, Q.H. DNA derived fluorescent bio-dots for sensitive detection of mercury and silver ions in aqueous solution. Appl. Surf. Sci. 2015, 347, 505–513. [Google Scholar] [CrossRef]
- Dang, D.K.; Chandrasekaran, S.; Ngo, Y.L.T.; Chung, J.S.; Kim, E.J.; Hur, S.H. One pot solid-state synthesis of highly fluorescent N and S co-doped carbon dots and its use as fluorescent probe for Ag+ detection in aqueous solution. Sens. Actuators B-Chem. 2018, 255, 3284–3291. [Google Scholar] [CrossRef]
- Li, T.Z.; Shuang, E.; Wang, J.H.; Chen, X.W. Regulating the properties of carbon dots via a solvent-involved molecule fusion strategy for improved sensing selectivity. Anal. Chim. Acta 2019, 1088, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Zhou, K.; Qiu, Y.; Xia, L.; Xia, Z.N.; Zhang, K.L.; Fu, Q. Strongly emissive formamide-derived N-doped carbon dots embedded Eu (III)-based metal-organic frameworks as a ratiometric fluorescent probe for ultrasensitive and visual quantitative detection of Ag. Sens. Actuators B-Chem. 2021, 339, 129922. [Google Scholar] [CrossRef]
- Guo, J.Q.; Ye, S.; Li, H.; Song, J.; Qu, J. Novel carbon dots with dual excitation for imaging and silver ion detection in living cells. Dye. Pigment. 2020, 183, 108723. [Google Scholar] [CrossRef]
- Shen, L.M.; Chen, M.L.; Hu, L.L.; Chen, X.W.; Wang, J.H. Growth and Stabilization of Silver Nanoparticles on Carbon Dots and Sensing Application. Langmuir 2013, 29, 16135–16140. [Google Scholar] [CrossRef]
- Zhao, X.E.; Lei, C.H.; Gao, Y.; Gao, H.; Zhu, S.Y.; Yang, X.; You, J.; Wang, H. A ratiometric fluorescent nanosensor for the detection of silver ions using graphene quantum dots. Sens. Actuators B-Chem. 2017, 253, 239–246. [Google Scholar] [CrossRef]
- Wu, H.F.; Tong, C.L. Ratiometric fluorometric determination of silver(I) by using blue-emitting silicon- and nitrogen-doped carbon quantum dots and red-emitting N-acetyl-L-cysteine-capped CdTe quantum dots. Microchim. Acta 2019, 186, 723. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.L.; Jha, S.; Basu, H.; Singhal, R.K.; Sharma, P.K.; Kailasa, S.K. Microwave-assisted synthesis of water-soluble Eu3+ hybrid carbon dots with enhanced fluorescence for the sensing of Hg2+ ions and imaging of fungal cells. New J. Chem. 2018, 42, 6125–6133. [Google Scholar] [CrossRef]
- Nolan, E.M.; Lippard, S.J. Tools and tactics for the optical detection of mercuric ion. Chem. Rev. 2008, 108, 3443–3480. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, H.; Jorge, P.A.S.; Fernandes, J.R.A.; da Silva, J.C.G.E. Hg(II) sensing based on functionalized carbon dots obtained by direct laser ablation. Sens. Actuators B-Chem. 2010, 145, 702–707. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, H.M.R.; Duarte, A.J.; da Silva, J.C.G.E. Optical fiber sensor for Hg(II) based on carbon dots. Biosens. Bioelectron. 2010, 26, 1302–1306. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.Z.; Chen, W. Nitrogen-doped carbon quantum dots: Facile synthesis and application as a ‘turn-off’ fluorescent probe for detection of Hg2+ ions. Biosens. Bioelectron. 2014, 55, 83–90. [Google Scholar] [CrossRef]
- Yang, M.; Liu, C.; Peng, Y.; Xiao, R.Z.; Zhang, S.; Zhang, Z.L.; Zhang, B.; Pang, D.-W. Surface chemistry tuning the selectivity of carbon nanodots towards Hg2+ recognition. Anal. Chim. Acta 2021, 1146, 33–40. [Google Scholar] [CrossRef]
- Wu, H.F.; Tong, C.L. Dual-Emission Fluorescent Probe for the Simultaneous Detection of Nitrite and Mercury(II) in Environmental Water Samples Based on the Tb3+-Modified Carbon Quantum Dot/3-Aminophenylboronic Acid Hybrid. Anal. Chem. 2020, 92, 8859–8866. [Google Scholar] [CrossRef]
- Liu, X.J.; Wang, C.Z.; Hupalo, M.; Yao, Y.X.; Tringides, M.C.; Lu, W.C.; Ho, K.M. Adsorption and growth morphology of rare-earth metals on graphene studied by ab initio calculations and scanning tunneling microscopy. Phys. Rev. B 2010, 82, 245408. [Google Scholar] [CrossRef]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence, 2nd ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2012. [Google Scholar]
- He, H.; Ma, Y.; Li, J.F.; Lai, X.F.; Chen, X.; Wang, L.; Zhang, W.; Huang, Y.; Zhang, P. Free regulation of luminous color of Ln-CQDs synthesized with citric acid-chelated Ln ions as the precursor. J. Lumin. 2020, 221, 117006. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef] [Green Version]
- Park, B.; Kim, S.J.; Lim, J.; Some, S.; Park, J.E.; Kim, S.J.; Kim, C.; Lee, T.J.; Jun, S.C. Tunable wide blue photoluminescence with europium decorated graphene. J. Mater. Chem. C 2015, 3, 4030–4038. [Google Scholar] [CrossRef]
- Wang, D.D.; Gao, H.; Roze, E.; Qu, K.; Liu, W.J.; Shao, Y.; Xin, S.; Wang, Y. Synthesis and photoluminescence of three-dimensional europium-complexed graphene macroassembly. J. Mater. Chem. C 2013, 1, 5772–5778. [Google Scholar] [CrossRef]
- Holá, K.; Sudolská, M.; Kalytchuk, S.; Nachtigallová, D.; Rogach, A.L.; Otyepka, M.; Zbořil, R. Graphitic Nitrogen Triggers Red Fluorescence in Carbon Dots. ACS Nano 2017, 11, 12402–12410. [Google Scholar] [CrossRef]
- Lin, L.P.; Song, X.H.; Chen, Y.Y.; Rong, M.C.; Wang, Y.R.; Zhao, L.; Zhao, T.; Chen, X. Europium-decorated graphene quantum dots as a fluorescent probe for label-free, rapid and sensitive detection of Cu2+ and L-cysteine. Anal. Chim. Acta 2015, 891, 261–268. [Google Scholar] [CrossRef]
- Stevens, J.S.; de Luca, A.C.; Pelendritis, M.; Terenghi, G.; Downes, S.; Schroeder, S.L.M. Quantitative analysis of complex amino acids and RGD peptides by X-ray photoelectron spectroscopy (XPS). Surf. Interface Anal. 2013, 45, 1238–1246. [Google Scholar] [CrossRef] [Green Version]
- Xia, D.D.; Li, J.F.; Li, W.Z.; Jiang, L.J.; Li, G.M. Lanthanides-based multifunctional luminescent films for ratiometric humidity sensing, information storage, and colored coating. J. Lumin. 2021, 231, 117784. [Google Scholar] [CrossRef]
- Idriss, H. On the wrong assignment of the XPS O1s signal at 531–532 eV attributed to oxygen vacancies in photo- and electro-catalysts for water splitting and other materials applications. Surf. Sci. 2021, 712, 121894. [Google Scholar] [CrossRef]
- Strauss, V.; Margraf, J.T.; Dolle, C.; Butz, B.; Nacken, T.J.; Walter, J.; Bauer, W.; Peukert, W.; Spiecker, E.; Clark, T.; et al. Carbon Nanodots: Toward a Comprehensive Understanding of Their Photoluminescence. J. Am. Chem. Soc. 2014, 136, 17308–17316. [Google Scholar] [CrossRef]
- Yamase, T.; Kobayashi, T.; Sugeta, M.; Naruke, H. Europium(III) luminescence and intramolecular energy transfer studies of polyoxometalloeuropates. J. Phys. Chem. A 1997, 101, 5046–5053. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Yang, J.; Li, H.R. Multiple color emission of solid-state hybrid material containing carbon dots and Europium(III) complexes. J. Lumin. 2020, 220, 116959. [Google Scholar] [CrossRef]
- Dexter, D.L. A Theory of Sensitized Luminescence in Solids. J. Chem. Phys. 1953, 21, 836–850. [Google Scholar] [CrossRef]
- Li, R.S.; Liu, J.H.; Yang, T.; Gao, P.F.; Wang, J.; Liu, H.; Zhen, S.J.; Li, Y.F.; Huang, C.Z. Carbon Quantum Dots-Europium(III) Energy Transfer Architecture Embedded in Electrospun Nanofibrous Membranes for Fingerprint Security and Document Counterspy. Anal. Chem. 2019, 91, 11185–11191. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.I.M.; Mariz, I.F.A.; Pinto, S.N.; Goncalves, G.; Bdikin, I.; Marques, P.A.A.P.; Neves, M.G.P.M.S.; Martinho, J.M.G.; Maçôas, E.M.S. Selective two-photon absorption in carbon dots: A piece of the photoluminescence emission puzzle. Nanoscale 2018, 10, 12505–12514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essner, J.B.; Kist, J.A.; Polo-Parada, L.; Baker, G.A. Artifacts and Errors Associated with the Ubiquitous Presence of Fluorescent Impurities in Carbon Nanodots. Chem. Mater. 2018, 30, 1878–1887. [Google Scholar] [CrossRef]
- Gupta, B.K.; Thanikaivelan, P.; Narayanan, T.N.; Song, L.; Gao, W.; Hayashi, T.; Reddy, A.L.M.; Saha, A.; Shanker, V.; Endo, M.; et al. Optical Bifunctionality of Europium-Complexed Luminescent Graphene Nanosheets. Nano Lett. 2011, 11, 5227–5233. [Google Scholar] [CrossRef]
- Bartolomei, M.; Pirani, F.; Marques, J.M.C. Aggregation enhancement of coronene molecules by seeding with alkali-metal ions. Phys. Chem. Chem. Phys. 2019, 21, 16005–16016. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic-Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. A 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Wu, P.; Hou, X.D.; Xu, J.J.; Chen, H.Y. Ratiometric fluorescence, electrochemiluminescence, and photoelectrochemical chemo/biosensing based on semiconductor quantum dots. Nanoscale 2016, 8, 8427–8442. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Baltimore, MD, USA, 2006. [Google Scholar]
- Martinho, J.M.G. Heavy-Atom Quenching of Monomer and Excimer Pyrene Fluorescence. J. Phys. Chem. 1989, 93, 6687–6692. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, C.; Martinho, J.; Maçôas, E. A Fluorescent Nanosensor for Silver (Ag+) and Mercury (Hg2+) Ions Using Eu (III)-Doped Carbon Dots. Nanomaterials 2022, 12, 385. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12030385
Correia C, Martinho J, Maçôas E. A Fluorescent Nanosensor for Silver (Ag+) and Mercury (Hg2+) Ions Using Eu (III)-Doped Carbon Dots. Nanomaterials. 2022; 12(3):385. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12030385
Chicago/Turabian StyleCorreia, Cátia, José Martinho, and Ermelinda Maçôas. 2022. "A Fluorescent Nanosensor for Silver (Ag+) and Mercury (Hg2+) Ions Using Eu (III)-Doped Carbon Dots" Nanomaterials 12, no. 3: 385. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12030385