Characterization of Silver Nanowire-Based Transparent Electrodes Obtained Using Different Drying Methods
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
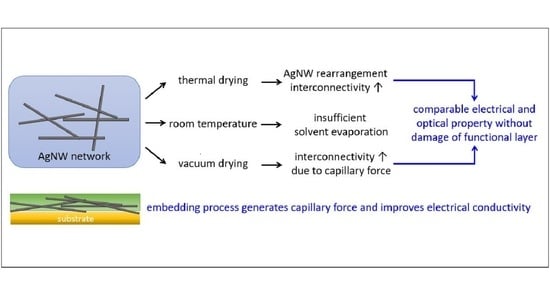
3.1. Morphological Characteristics
3.2. Electrical Characteristics
3.3. Optical Characteristics
3.4. Device Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoo, J.J.; Wieghold, S.; Sponseller, M.C.; Chua, M.R.; Bertram, S.N.; Hartono, N.T.P.; Tresback, J.S.; Hansen, E.C.; Correa-Baena, J.-P.; Bulović, V.; et al. An interface stabilized perovskite solar cell with high stabilized efficiency and low voltage loss. Energy Environ. Sci. 2019, 12, 2192–2199. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.; Jee, E.; Choi, D.; Kim, W.; Kim, J.S.; Amoli, V.; Sung, T.; Choi, D.; Kim, D.H.; Kwon, J.-Y. Ultrasensitive, low-power oxide transistor-based mechanotransducer with microstructured, deformable ionic dielectrics. ACS Appl. Mater. Interfaces 2018, 10, 31472–31479. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Jo, J.-W.; Kim, Y.-J.; Choi, S.; Kwon, S.M.; Jeon, S.P.; Facchetti, A.; Kim, Y.-H.; Park, S.K. Corrugated heterojunction metal-oxide thin-film transistors with high electron mobility via vertical interface manipulation. Adv. Mater. 2018, 30, 1804120. [Google Scholar] [CrossRef] [PubMed]
- Rim, Y.S.; Chen, H.; Zhu, B.; Bae, S.-H.; Zhu, S.; Li, P.J.; Wang, I.C.; Yang, Y. Interface engineering of metal oxide semiconductors for biosensing applications. Adv. Mater. Interfaces 2017, 4, 1700020. [Google Scholar]
- Song, H.; Ma, Y.; Ko, D.; Jo, S.; Hyun, D.C.; Kim, C.S.; Oh, H.-J.; Kim, J. Influence of humidity for preparing sol-gel ZnO layer: Characterization and optimization for optoelectronic device applications. Appl. Surf. Sci. 2020, 512, 145660. [Google Scholar] [CrossRef]
- Ko, D.; Gu, B.; Cheon, J.; Roh, J.-S.; Kim, C.S.; Jo, S.; Hyun, D.C.; Kim, J. Decoupling the contributions to the enhancement of electrical conductivity in transparent silver nanowire/zinc oxide composite electrodes. Mater. Chem. Phys. 2019, 223, 634–640. [Google Scholar] [CrossRef]
- Lv, H.; Yang, Z.; Wang, P.L.; Ji, G.; Song, J.; Zheng, L.; Zeng, H.; Xu, Z.J. A voltage-boosting strategy enabling a low-frequency flexible electromagnetic wave absorption device. Adv. Mater. 2018, 30, 1706343. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, D.; Tang, Z.; Liang, G.; Yang, Q.; Li, H.; Ma, L.; Mo, F.; Zhi, C. A mechanically durable and device-level tough Zn-MnO2 Battery with high flexibility. Energy Storage Mater. 2019, 23, 636–645. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Huang, Y.; Xie, J.; Zhao, X.; Li, C.; Qu, J.; Zhang, Q.; Sung, J.; He, B.; et al. 3D printing fiber electrodes for an all-fiber integrated electronic device via hybridization of an asymmetric supercapacitor and temperature sensor. Adv. Sci. 2018, 5, 1801114. [Google Scholar] [CrossRef]
- Wang, X.; Guo, X.; Ye, J.; Zheng, N.; Kohli, P.; Choi, D.; Zhang, Y.; Xie, Z.; Zhang, Q.; Luan, H.; et al. Freestanding 3D mesostructures, functional devices and shape-programmable systems based on mechanically induced assembly with shape memory polymers. Adv. Mater. 2019, 31, 1805615. [Google Scholar] [CrossRef]
- Xu, K.; Lu, Y.; Takei, K. Multifunctional skin-inspired flexible sensory systems for wearable electronics. Adv. Mater. Technol. 2019, 4, 1800628. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Cong, S.; Tian, Z.; Song, Y.; Yu, L.; Lu, C.; Shao, Y.; Li, J.; Zou, G.; Rümmeli, M.H.; et al. Flexible perovskite solar cell-driven photo-rechargeable lithium-ion capacitor for self-powered wearable strain sensors. Nano Energy 2019, 60, 247–256. [Google Scholar] [CrossRef]
- Scalia, A.; Bella, F.; Lamberti, A.; Bianco, S.; Gerbaldi, C.; Tresso, E.; Pirri, C.F. A flexible and portable powerpack by solid-state supercapacitor and dye-sensitized solar cell integration. J. Power Sources 2017, 359, 311–321. [Google Scholar] [CrossRef]
- Lin, S.; Bai, X.; Wang, H.; Wang, H.; Song, J.; Huang, K.; Wang, C.; Wang, N.; Li, B.; Lei, M.; et al. Roll-to-roll production of transparent silver-nanofiber-network electrodes for flexible electrochromic smart windows. Adv. Mater. 2017, 29, 1703238. [Google Scholar] [CrossRef] [PubMed]
- Mallikarjuna, K.; Kim, H. Highly transparent conductive reduced graphene oxide/silver nanowires/silver grid electrodes for low-voltage electrochromic smart windows. ACS Appl. Mater. Interfaces 2019, 11, 1969–1978. [Google Scholar] [CrossRef]
- Huang, S.; Liu, Y.; Zhao, Y.; Ren, Z.; Guo, C.F. Flexible electronics: Stretchable electrodes and their future. Adv. Funct. Mater. 2019, 29, 1805924. [Google Scholar] [CrossRef]
- Dong, L.; Liang, G.; Xu, C.; Liu, W.; Pan, Z.-Z.; Zhou, E.; Kang, F.; Yang, Q.-H. Multi hierarchical construction-induced superior capacitive performances of flexible electrodes for wearable energy storage. Nano Energy 2017, 34, 242–248. [Google Scholar] [CrossRef]
- Aliprandi, A.; Moreira, T.; Anichini, C.; Stoeckel, M.-A.; Eredia, M.; Sassi, U.; Bruna, M.; Pinheiro, C.; Laia, C.A.T.; Boncacchi, S.; et al. Hybrid-copper-nanowire-reduced-graphene-oxide coatings: A “Green Solution” toward highly transparent, highly conductive, and flexible electrodes for (opto)electronics. Adv. Mater. 2017, 29, 1703225. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Chang, M.; Meng, L.; Wan, X.; Gao, H.; Zhang, Y.; Zhao, K.; Sun, Z.; Li, C.; Liu, S.; et al. Flexible organic photovoltaics based on water-processed silver nanowire electrodes. Nat. Electron. 2019, 2, 513–520. [Google Scholar] [CrossRef]
- Ko, D.; Gu, B.; Kang, S.J.; Jo, S.; Hyun, D.C.; Kim, C.S.; Kim, J. Critical work of adhesion for economical patterning of silver nanowire-based transparent electrodes. J. Mater. Chem. A 2019, 7, 14536–14544. [Google Scholar] [CrossRef]
- Shajari, S.; Ramakrishnan, S.; Karan, K.; Sudak, L.J.; Sundararaj, U. Ultrasensitive wearable sensor with novel hybrid structures of silver nanowires and carbon nanotubes in fluoroelastomer: Multi-directional sensing for human health monitoring and stretchable electronics. Appl. Mater. Today 2022, 26, 101295. [Google Scholar] [CrossRef]
- Sannicolo, T.; Chae, W.H.; Mwaura, J.; Bulovic, V.; Grossman, J.C. Silver nanowire back electrode stabilized with graphene oxide encapsulation for inverted semitransparent organic solar cells with longer lifetime. ACS Appl. Energy Mater. 2021, 4, 1431. [Google Scholar] [CrossRef]
- Luo, Y.; Ning, T.; Pei, Y.; Feng, X.; Zhang, S.; Lu, B.; Wang, L. High-performance and tailored honeycombed Ag nanowire networks fabricated by a novel electrospray assisted etching process. Appl. Surf. Sci. 2022, 571, 151081. [Google Scholar] [CrossRef]
- Huang, Q.; Zhu, Y. Patterning of metal nanowire networks: Methods and applications. ACS Appl. Mater. Interfaces 2021, 13, 60736. [Google Scholar] [CrossRef]
- Corletto, A.; Shapter, J.G. Nanoscale patterning of carbon nanotubes: Techniques, applications, and future. Adv. Sci. 2021, 8, 2001778. [Google Scholar] [CrossRef]
- Kim, K.; Ha, M.; Choi, B.; Joo, S.H.; Kang, H.S.; Park, J.H.; Gu, B.; Park, C.; Park, C.; Kim, J.; et al. Biodegradable, electro-active chitin nanofiber films for flexible piezoelectric transducers. Nano Energy 2018, 48, 275–283. [Google Scholar] [CrossRef]
- Leem, D.-S.; Edwards, A.; Faist, M.; Nelson, J.; Bradley, D.D.; Mello, J.C. Efficient organic solar cells with solution-processed silver nanowire electrodes. Adv. Mater. 2011, 23, 4371–4375. [Google Scholar] [CrossRef]
- Chen, D.; Liang, J.; Liu, C.; Saldanha, G.; Zhao, F.; Tong, K.; Liu, J.; Pei, Q. Thermally stable silver nanowire-polyimide transparent electrode based on atomic layer deposition of zinc oxide on silver nanowires. Adv. Funct. Mater. 2015, 25, 7512–7520. [Google Scholar] [CrossRef]
- Song, T.-B.; Rim, Y.S.; Liu, F.; Bob, B.; Ye, S.; Hsieh, Y.-T.; Yang, Y. Highly robust silver nanowire network for transparent electrode. ACS Appl. Mater. Interfaces 2015, 7, 24601–24607. [Google Scholar] [CrossRef]
- Liu, G.-S.; Xu, Y.; Kong, Y.; Wang, L.; Wang, J.; Xie, X.; Luo, Y.; Yang, B.-R. Comprehensive stability improvement of silver nanowire networks via self-assembled mercapto inhibitors. ACS Appl. Mater. Interfaces 2018, 10, 37699–37708. [Google Scholar] [CrossRef]
- Liu, G.S.; He, M.; Wang, T.; Wang, L.; He, Z.; Zhan, R.; Chen, L.; Chen, Y.; Yang, B.-R.; Luo, Y.; et al. Optically programmable plateau-rayleigh instability for high-resolution and scalable morphology manipulation of silver nanowires for flexible optoelectronics. ACS Appl. Mater. Interfaces 2020, 12, 53984–53993. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Gao, X.-M.; Bi, Y.-G.; Zhang, X.-L.; Yin, D.; Wen, X.-M.; Liu, Y.-F.; Feng, J.; Sun, H.-B. PFSA-passivated silver nanowire transparent electrodes for highly flexible organic-light-emitting devices with improved stability. Org. Electron. 2020, 84, 105727. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, S.B.; Ko, D.; Jung, J.; Jo, S.; Hyun, D.C.; Oh, H.-J.; Kim, J. Characterization of Silver Nanowire-Based Transparent Electrodes Obtained Using Different Drying Methods. Nanomaterials 2022, 12, 461. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12030461
Chu SB, Ko D, Jung J, Jo S, Hyun DC, Oh H-J, Kim J. Characterization of Silver Nanowire-Based Transparent Electrodes Obtained Using Different Drying Methods. Nanomaterials. 2022; 12(3):461. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12030461
Chicago/Turabian StyleChu, Seo Bum, Dongwook Ko, Jinwook Jung, Sungjin Jo, Dong Choon Hyun, Hyeon-Ju Oh, and Jongbok Kim. 2022. "Characterization of Silver Nanowire-Based Transparent Electrodes Obtained Using Different Drying Methods" Nanomaterials 12, no. 3: 461. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12030461