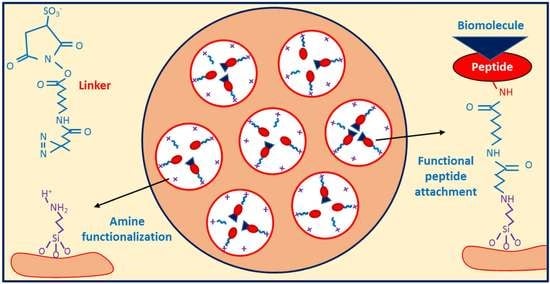
Strategy for Conjugating Oligopeptides to Mesoporous Silica Nanoparticles Using Diazirine-Based Heterobifunctional Linkers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Mesoporous Silica Nanoparticles (MSNPs) Synthesis
2.3. Amine Functionalization and Quantification
2.4. Peptide Attachment to MSNPAs
2.5. Material Characterization
2.6. Quantification of Peptide Attachment
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, H.; Yuan, B.; Zhang, X.; Scherman, O.A. Supramolecular chemistry at interfaces: Host–guest interactions for fabricating multifunctional biointerfaces. Acc. Chem. Res. 2014, 47, 2106–2115. [Google Scholar] [CrossRef] [PubMed]
- Houk, K.N.; Leach, A.G.; Kim, S.P.; Zhang, X. Binding Affinities of Host–Guest, Protein–Ligand, and Protein–Transition-State Complexes. Angew. Chem. Int. Ed. 2003, 42, 4872–4897. [Google Scholar] [CrossRef]
- Uhlenheuer, D.A.; Petkau, K.; Brunsveld, L. Combining supramolecular chemistry with biology. Chem. Soc. Rev. 2010, 39, 2817–2826. [Google Scholar] [CrossRef] [PubMed]
- Hage, D.S. Analysis of biological interactions by affinity chromatography: Clinical and pharmaceutical applications. Clin. Chem. 2017, 63, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Frączyk, J.; Walczak, M.; Kamiński, Z.J. Cellulose template assembled synthetic peptides as molecular receptors. Curr. Protein Peptide Sci. 2016, 17, 117–126. [Google Scholar] [CrossRef]
- Menegatti, S. Peptoid Affinity Ligands. U.S. Patent 10,065,988 B2, 4 September 2018. [Google Scholar]
- Tozzi, C.; Anfossi, L.; Giraudi, G. Affinity chromatography techniques based on the immobilisation of peptides exhibiting specific binding activity. J. Chromatogr. B 2003, 797, 289–304. [Google Scholar] [CrossRef]
- Tothill, I.E. Peptides as molecular receptors. In Recognition Receptors in Biosensors; Springer: Berlin/Heidelberg, Germany, 2010; pp. 249–274. [Google Scholar]
- Noppe, W.; Plieva, F.M.; Galaev, I.Y.; Vanhoorelbeke, K.; Mattiasson, B.; Deckmyn, H. Immobilised peptide displaying phages as affinity ligands: Purification of lactoferrin from defatted milk. J. Chromatogr. A 2006, 1101, 79–85. [Google Scholar] [CrossRef]
- Pan, L.; He, Q.; Liu, J.; Chen, Y.; Ma, M.; Zhang, L.; Shi, J. Nuclear-Targeted Drug Delivery of TAT Peptide-Conjugated Monodisperse Mesoporous Silica Nanoparticles. J. Am. Chem. Soc. 2012, 134, 5722–5725. [Google Scholar] [CrossRef]
- Song, N.; Yang, Y.-W. Molecular and supramolecular switches on mesoporous silica nanoparticles. Chem. Soc. Rev. 2015, 44, 3474–3504. [Google Scholar] [CrossRef]
- Moradipour, M.; Chase, E.K.; Khan, M.A.; Asare, S.O.; Lynn, B.C.; Rankin, S.E.; Knutson, B.L. Interaction of lignin-derived dimer and eugenol-functionalized silica nanoparticles with supported lipid bilayers. Colloids Surf. B Biointerfaces 2020, 191, 111028. [Google Scholar] [CrossRef]
- Ma, Y.; Qi, L.; Ma, J.; Wu, Y.; Liu, O.; Cheng, H. Large-pore mesoporous silica spheres: Synthesis and application in HPLC. Colloids Surf. A 2003, 229, 1–8. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, F.; Fu, Y.; Jin, W.; Yang, P.; Zhao, D. Biomolecule separation using large pore mesoporous SBA-15 as a substrate in high performance liquid chromatography. Chem. Commun. 2002, 2, 752–753. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Bioconjugate Techniques; Academic Press: London, UK, 2013. [Google Scholar]
- Farkaš, P.; Bystrický, S. Chemical conjugation of biomacromolecules: A mini-review. Chem. Pap. 2010, 64, 683–695. [Google Scholar] [CrossRef]
- Sundoro, B.M. Bifunctional Linker. U.S. Patent 4,680,338, 14 July 1987. [Google Scholar]
- Shi, J.-M.; Pei, J.; Liu, E.-Q.; Zhang, L. Bis (sulfosuccinimidyl) suberate (BS3) crosslinking analysis of the behavior of amyloid-β peptide in solution and in phospholipid membranes. PLoS ONE 2017, 12, e0173871. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Xiao, S.-J.; Guo, D.-J.; Wang, J.; Chao, J.; Liu, H.-B.; Pei, J.; Chen, Y.-Q.; Tang, Y.-C.; Liu, J.-N. Biofunctionalisation of porous silicon (PS) surfaces by using homobifunctional cross-linkers. J. Mater. Chem. 2006, 16, 570–578. [Google Scholar] [CrossRef]
- Lee, J.P.; Kassianidou, E.; MacDonald, J.I.; Francis, M.B.; Kumar, S. N-terminal specific conjugation of extracellular matrix proteins to 2-pyridinecarboxaldehyde functionalized polyacrylamide hydrogels. Biomaterials 2016, 102, 268–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, C.Y.; Owens, N.A.; Wampler, R.D.; Ying, Y.; Granger, J.H.; Porter, M.D.; Takahashi, M.; Shimazu, K. Succinimidyl Ester Surface Chemistry: Implications of the Competition between Aminolysis and Hydrolysis on Covalent Protein Immobilization. Langmuir 2014, 30, 12868–12878. [Google Scholar] [CrossRef]
- Kalkhof, S.; Sinz, A. Chances and pitfalls of chemical cross-linking with amine-reactive N-hydroxysuccinimide esters. Anal. Bioanal. Chem. 2008, 392, 305–312. [Google Scholar] [CrossRef]
- Karakeçili, A.G.; Satriano, C.; Gümüşderelioğlu, M.; Marletta, G. Enhancement of fibroblastic proliferation on chitosan surfaces by immobilized epidermal growth factor. Acta Biomater. 2008, 4, 989–996. [Google Scholar] [CrossRef]
- Li, B.; Chen, J.; Wang, J.H.C. RGD peptide-conjugated poly (dimethylsiloxane) promotes adhesion, proliferation, and collagen secretion of human fibroblasts. J. Biomed. Mater. Res. A 2006, 79, 989–998. [Google Scholar] [CrossRef]
- Oluwabusola, E. Development of Diazirine-Based Crosslinking Agents for Covalently Linking Protein. Master’s Thesis, University of Salford, Manchester, UK, 2015. [Google Scholar]
- Shigdel, U.K.; Zhang, J.; He, C. Diazirine-Based DNA Photo-Cross-Linking Probes for the Study of Protein–DNA Interactions. Angew. Chem. Int. Ed. 2008, 47, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Chim, L.K.-L. Immobilizing Gradients of Neurotrophic Factors for Directed Peripheral Nervous System Cell Migration Guidance. Master’s Thesis, Johns Hopkins University, Baltimore, MD, USA, 2016. [Google Scholar]
- Das, J. Aliphatic diazirines as photoaffinity probes for proteins: Recent developments. Chem. Rev. 2011, 111, 4405–4417. [Google Scholar] [CrossRef] [PubMed]
- Suchanek, M.; Radzikowska, A.; Thiele, C. Photo-leucine and photo-methionine allow identification of protein-protein interactions in living cells. Nat. Meth. 2005, 2, 261. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kohler, J.J. Photoactivatable crosslinking sugars for capturing glycoprotein interactions. J. Am. Chem. Soc. 2008, 130, 3278–3279. [Google Scholar] [CrossRef]
- Liashkovich, I.; Rosso, G.; Rangl, M.; Ebner, A.; Hafezi, W.; Kühn, J.; Schön, P.; Hinterdorfer, P.; Shahin, V. Photopicking: In Situ Approach for Site-Specific Attachment of Single Multiprotein Nanoparticles to Atomic Force Microscopy Tips. Adv. Func. Mater. 2017, 27, 1604506. [Google Scholar] [CrossRef]
- Moussus, M.; der Loughian, C.; Fuard, D.; Courçon, M.; Gulino-Debrac, D.; Delanoë-Ayari, H.; Nicolas, A. Intracellular stresses in patterned cell assemblies. Soft Matter 2014, 10, 2414–2423. [Google Scholar] [CrossRef]
- Lozito, T.P.; Tuan, R.S. Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J. Cell. Physiol. 2011, 226, 385–396. [Google Scholar] [CrossRef]
- Lu, J.; Shen, H.-H.; Wu, Z.; Wang, B.; Zhao, D.; He, L. Self-assembly of bi-functional peptides on large-pore mesoporous silica nanoparticles for miRNA binding and delivery. J. Mater. Chem. B 2015, 3, 7653–7657. [Google Scholar] [CrossRef]
- Terracciano, R.; Casadonte, F.; Pasqua, L.; Candeloro, P.; Di Fabrizio, E.; Urbani, A.; Savino, R. Enhancing plasma peptide MALDI-TOF-MS profiling by mesoporous silica assisted crystallization. Talanta 2010, 80, 1532–1538. [Google Scholar] [CrossRef]
- Liu, H.-J.; Xu, P. Smart Mesoporous Silica Nanoparticles for Protein Delivery. Nanomaterials 2019, 9, 511. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Lei, C.; Yu, C. Mesoporous Silica Nanoparticles for Protein Protection and Delivery. Front. Chem. 2019, 7, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlipf, D.M.; Zhou, S.; Khan, M.A.; Rankin, S.E.; Knutson, B.L. Effects of Pore Size and Tethering on the Diffusivity of Lipids Confined in Mesoporous Silica. Adv. Mater. Interfaces 2017, 4, 1601103. [Google Scholar] [CrossRef]
- Yushkova, E.D.; Nazarova, E.A.; Matyuhina, A.V.; Noskova, A.O.; Shavronskaya, D.O.; Vinogradov, V.V.; Skvortsova, N.N.; Krivoshapkina, E.F. Application of Immobilized Enzymes in Food Industry. J. Agric. Food Chem. 2019, 67, 11553–11567. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, J.M.; Nidetzky, B. The Microenvironment in Immobilized Enzymes: Methods of Characterization and Its Role in Determining Enzyme Performance. Molecules 2019, 24, 3460. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, S.; Chakravarty, A.; Ikram, S. A comprehensive review on incredible renewable carriers as promising platforms for enzyme immobilization & thereof strategies. Int. J. Biol. Macromol. 2021, 167, 962–986. [Google Scholar] [CrossRef]
- Castillo, R.R.; Lozano, D.; Vallet-Regí, M. Mesoporous Silica Nanoparticles as Carriers for Therapeutic Biomolecules. Pharmaceutics 2020, 12, 432. [Google Scholar] [CrossRef]
- Kumar, P.; Tambe, P.; Paknikar, K.M.; Gajbhiye, V. Mesoporous silica nanoparticles as cutting-edge theranostics: Advancement from merely a carrier to tailor-made smart delivery platform. J. Control. Release 2018, 287, 35–57. [Google Scholar] [CrossRef]
- Wilkins, D.K.; Grimshaw, S.B.; Receveur, V.; Dobson, C.M.; Jones, J.A.; Smith, L.J. Hydrodynamic Radii of Native and Denatured Proteins Measured by Pulse Field Gradient NMR Techniques. Biochemistry 1999, 38, 16424–16431. [Google Scholar] [CrossRef]
- Khan, M.A.; Wallace, W.T.; Islam, S.Z.; Nagpure, S.; Strzalka, J.; Littleton, J.M.; Rankin, S.E.; Knutson, B.L. Adsorption and Recovery of Polyphenolic Flavonoids Using TiO2-Functionalized Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 32114–32125. [Google Scholar] [CrossRef]
- Kim, T.-W.; Chung, P.-W.; Lin, V.S.Y. Facile Synthesis of Monodisperse Spherical MCM-48 Mesoporous Silica Nanoparticles with Controlled Particle Size. Chem. Mater. 2010, 22, 5093–5104. [Google Scholar] [CrossRef]
- Tan, B.; Rankin, S.E. Interfacial Alignment Mechanism of Forming Spherical Silica with Radially Oriented Nanopores. J. Phys. Chem. B 2004, 108, 20122–20129. [Google Scholar] [CrossRef]
- Gu, J.; Huang, K.; Zhu, X.; Li, Y.; Wei, J.; Zhao, W.; Liu, C.; Shi, J. Sub-150 nm mesoporous silica nanoparticles with tunable pore sizes and well-ordered mesostructure for protein encapsulation. J. Colloid Interface Sci. 2013, 407, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Ujiie, H.; Urata, C.; Yamamoto, E.; Yamauchi, Y.; Kuroda, K. A multifunctional role of trialkylbenzenes for the preparation of aqueous colloidal mesostructured/mesoporous silica nanoparticles with controlled pore size, particle diameter, and morphology. Nanoscale 2015, 7, 19557–19567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berneschi, S.; Baldini, F.; Cosci, A.; Farnesi, D.; Conti, G.N.; Tombelli, S.; Trono, C.; Pelli, S.; Giannetti, A. Fluorescence biosensing in selectively photo–activated microbubble resonators. Sens. Actuators B 2017, 242, 1057–1064. [Google Scholar] [CrossRef]
- Lozito, T.P.; Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Human mesenchymal stem cells generate a distinct pericellular zone of MMP activities via binding of MMPs and secretion of high levels of TIMPs. Matrix Biol. 2014, 34, 132–143. [Google Scholar] [CrossRef]
- ThermoFisher Scientific. Bioconjugation Technical Handbook; Thermo Fisher Scientific Inc.: Pittsburgh, PA, USA, 2018. [Google Scholar]
- Tozzi, C.; Anfossi, L.; Giraudi, G.; Giovannoli, C.; Baggiani, C.; Vanni, A. Chromatographic characterisation of an estrogen-binding affinity column containing tetrapeptides selected by a combinatorial-binding approach. J. Chromatogr. A 2002, 966, 71–79. [Google Scholar] [CrossRef]
- Iacobucci, C.; Götze, M.; Piotrowski, C.; Arlt, C.; Rehkamp, A.; Ihling, C.; Hage, C.; Sinz, A. Carboxyl-photo-reactive MS-cleavable cross-linkers: Unveiling a hidden aspect of diazirine-based reagents. Anal. Chem. 2018, 90, 2805–2809. [Google Scholar] [CrossRef]
- Ezzeddine, Z.; Batonneau-Gener, I.; Pouilloux, Y.; Hamad, H.; Saad, Z.; Kazpard, V. Divalent heavy metals adsorption onto different types of EDTA-modified mesoporous materials: Effectiveness and complexation rate. Microporous Mesoporous Mater. 2015, 212, 125–136. [Google Scholar] [CrossRef]
- Na, H.-K.; Kim, M.-H.; Park, K.; Ryoo, S.-R.; Lee, K.E.; Jeon, H.; Ryoo, R.; Hyeon, C.; Min, D.-H. Efficient Functional Delivery of siRNA using Mesoporous Silica Nanoparticles with Ultralarge Pores. Small 2012, 8, 1752–1761. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, N.; Kim, H.; Kim, J.; Choi, S.H.; Kim, J.H.; Kim, T.; Song, I.C.; Park, S.P.; Moon, W.K. Uniform mesoporous dye-doped silica nanoparticles decorated with multiple magnetite nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence imaging, and drug delivery. J. Am. Chem. Soc. 2009, 132, 552–557. [Google Scholar] [CrossRef]
- Schlipf, D.M.; Rankin, S.E.; Knutson, B.L. Selective external surface functionalization of large-pore silica materials capable of protein loading. Microporous Mesoporous Mater. 2017, 244, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Ritter, H.; Nieminen, M.; Karppinen, M.; Brühwiler, D. A comparative study of the functionalization of mesoporous silica MCM-41 by deposition of 3-aminopropyltrimethoxysilane from toluene and from the vapor phase. Microporous Mesoporous Mater. 2009, 121, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Mu, Q.; Jiang, G.; Chen, L.; Zhou, H.; Fourches, D.; Tropsha, A.; Yan, B. Chemical Basis of Interactions Between Engineered Nanoparticles and Biological Systems. Chem. Rev. 2014, 114, 7740–7781. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A. Nanoharvesting and Delivery of Bioactive Materials Using Engineered Silica Nanoparticles. Ph.D. Thesis, University of Kentucky, Lexington, KE, USA, 2019. [Google Scholar]
- Van Itallie, C.M.; Mitic, L.L.; Anderson, J.M. Claudin-2 forms homodimers and is a component of a high molecular weight protein complex. J. Biol. Chem. 2011, 286, 3442–3450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casanova, D.; Giaume, D.; Moreau, M.; Martin, J.-L.; Gacoin, T.; Boilot, J.-P.; Alexandrou, A. Counting the number of proteins coupled to single nanoparticles. J. Am. Chem. Soc. 2007, 129, 12592–12593. [Google Scholar] [CrossRef]
- Waddell, C. The Development of a Rapid Fiber-Based Immunoassay as a Point-of-Care or In-Home Diagnostic Test. Master’s Thesis, Clemson University, Clemson, SC, USA, 2013. [Google Scholar]
- Ettinger, R. Infrared Spectrum of Diazirine. J. Chem. Phys. 1964, 40, 1693–1699. [Google Scholar] [CrossRef]
- Gambi, A.; Winnewisser, M.; Christiansen, J.J. The infrared spectrum of diazirine: H2C<(N=N). Rovibrational analysis of the ν3 fundamental. J. Mol. Spectrosc. 1983, 98, 413–424. [Google Scholar] [CrossRef]
- Martucci, N.M.; Migliaccio, N.; Ruggiero, I.; Rea, I.; Terracciano, M.; De Stefano, L.; Arcari, P.; Rendina, I.; Lamberti, A. Bioengineered Surfaces for Real-Time Label-Free Detection of Cancer Cells. In Lab-on-a-Chip Fabrication and Application; IntechOpen: London, UK, 2016; p. 179. [Google Scholar]
- Mitchell, R.; Merritt, J. The infrared spectra of 3,3-dimethyldiazirine and 3,3-dimethyl-d6-diazirine. J. Mol. Spectrosc. 1968, 27, 197–209. [Google Scholar] [CrossRef]
- Barth, A. The infrared absorption of amino acid side chains. Prog. Biophys. Mol. Biol. 2000, 74, 141–173. [Google Scholar] [CrossRef]
- Kolev, T.; Spiteller, M.; Koleva, B. Spectroscopic and structural elucidation of amino acid derivatives and small peptides: Experimental and theoretical tools. Amino Acids 2010, 38, 45–50. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, B.; Han, F.; Zhou, Y.; Liu, H.; Li, Y.; Cui, L.; Tan, T.; Wang, N. Green synthesis, composition analysis and surface active properties of sodium cocoyl glycinate. Am. J. Anal. Chem. 2013, 4, 445. [Google Scholar] [CrossRef] [Green Version]
- Ainavarapu, S.R.K.; Brujić, J.; Huang, H.H.; Wiita, A.P.; Lu, H.; Li, L.; Walther, K.A.; Carrion-Vazquez, M.; Li, H.; Fernandez, J.M. Contour Length and Refolding Rate of a Small Protein Controlled by Engineered Disulfide Bonds. Biophys. J. 2007, 92, 225–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.A.; Wallace, W.T.; Sambi, J.; Rogers, D.T.; Littleton, J.M.; Rankin, S.E.; Knutson, B.L. Nanoharvesting of bioactive materials from living plant cultures using engineered silica nanoparticles. Mater. Sci. Eng. C 2020, 106, 110190. [Google Scholar] [CrossRef] [PubMed]
- Ejima, D.; Yumioka, R.; Tsumoto, K.; Arakawa, T. Effective elution of antibodies by arginine and arginine derivatives in affinity column chromatography. Anal. Biochem. 2005, 345, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Endo, T. Fractionation of glycoprotein-derived oligosaccharides by affinity chromatography using immobilized lectin columns. J. Chromatogr. A 1996, 720, 251–261. [Google Scholar] [CrossRef]
- Liau, C.Y.; Chang, T.M.; Pan, J.P.; Chen, W.L.; Mao, S.J. Purification of human plasma haptoglobin by hemoglobin-affinity column chromatography. J. Chromatogr. B 2003, 790, 209–216. [Google Scholar] [CrossRef]
- Hu, Y.; Cai, K.; Luo, Z.; Jandt, K.D. Layer-by-layer assembly of β-estradiol loaded mesoporous silica nanoparticles on titanium substrates and its implication for bone homeostasis. Adv. Mater. 2010, 22, 4146–4150. [Google Scholar] [CrossRef]
- Steffi, C.; Wang, D.; Kong, C.H.; Wang, Z.; Lim, P.N.; Shi, Z.; San Thian, E.; Wang, W. Estradiol-loaded poly (ε-caprolactone)/silk fibroin electrospun microfibers decrease osteoclast activity and retain osteoblast function. ACS Appl. Mater. Interfaces 2018, 10, 9988–9998. [Google Scholar] [CrossRef]
- Sardan, M.; Yildirim, A.; Mumcuoglu, D.; Tekinay, A.B.; Guler, M.O. Noncovalent functionalization of mesoporous silica nanoparticles with amphiphilic peptides. J. Mater. Chem. B 2014, 2, 2168–2174. [Google Scholar] [CrossRef]
- Sweeney, S.K.; Luo, Y.; O’Donnell, M.A.; Assouline, J.G. Peptide-mediated targeting mesoporous silica nanoparticles: A novel tool for fighting bladder cancer. J. Biomed. Nanotechnol. 2017, 13, 232–242. [Google Scholar] [CrossRef]
- Lahcen, A.A.; Baleg, A.A.; Baker, P.; Iwuoha, E.; Amine, A. Synthesis and electrochemical characterization of nanostructured magnetic molecularly imprinted polymers for 17-β-estradiol determination. Sens. Actuators B 2017, 241, 698–705. [Google Scholar] [CrossRef]





| Particle Type | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) a |
|---|---|---|---|
| MSNP | 729 | 2.32 | 7.9 ± 2.2 |
| MSNPA | 469 | 1.50 | 7.6 ± 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.A.; Ghanim, R.W.; Kiser, M.R.; Moradipour, M.; Rogers, D.T.; Littleton, J.M.; Bradley, L.H.; Lynn, B.C.; Rankin, S.E.; Knutson, B.L. Strategy for Conjugating Oligopeptides to Mesoporous Silica Nanoparticles Using Diazirine-Based Heterobifunctional Linkers. Nanomaterials 2022, 12, 608. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12040608
Khan MA, Ghanim RW, Kiser MR, Moradipour M, Rogers DT, Littleton JM, Bradley LH, Lynn BC, Rankin SE, Knutson BL. Strategy for Conjugating Oligopeptides to Mesoporous Silica Nanoparticles Using Diazirine-Based Heterobifunctional Linkers. Nanomaterials. 2022; 12(4):608. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12040608
Chicago/Turabian StyleKhan, Md Arif, Ramy W. Ghanim, Maelyn R. Kiser, Mahsa Moradipour, Dennis T. Rogers, John M. Littleton, Luke H. Bradley, Bert C. Lynn, Stephen E. Rankin, and Barbara L. Knutson. 2022. "Strategy for Conjugating Oligopeptides to Mesoporous Silica Nanoparticles Using Diazirine-Based Heterobifunctional Linkers" Nanomaterials 12, no. 4: 608. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12040608