Spectral and Structural Properties of High-Quality Reduced Graphene Oxide Produced via a Simple Approach Using Tetraethylenepentamine
Abstract
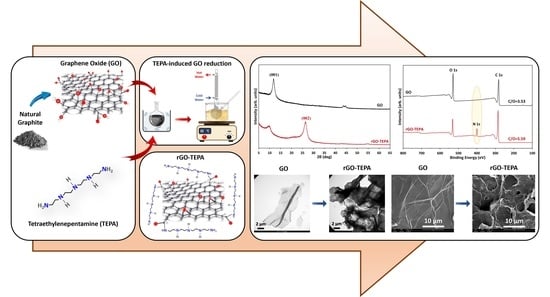
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. GO Nanosheet Preparation
2.3. GO Reduction and Functionalization
2.4. Characterization Techniques
3. Results and Discussion
3.1. FTIR and Raman Spectral Properties
3.2. Structural and Morphological Properties
3.3. XPS and Compositional Properties
3.4. Thermal Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurian, M. Recent Progress in the Chemical Reduction of Graphene Oxide by Green Reductants–A Mini Review. Carbon Trends 2021, 5, 100120. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.H.; Li, J.Y.; Tang, Z.R.; Xu, Y.J. 3D Graphene-Based Gel Photocatalysts for Environmental Pollutants Degradation. Environ. Pollut. 2019, 253, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Alkhouzaam, A.; Qiblawey, H. Functional GO-Based Membranes for Water Treatment and Desalination: Fabrication Methods, Performance and Advantages. A Review. Chemosphere 2021, 274, 129853. [Google Scholar] [CrossRef]
- Han, C.; Zhang, N.; Xu, Y.J. Structural Diversity of Graphene Materials and Their Multifarious Roles in Heterogeneous Photocatalysis. Nano Today 2016, 11, 351–372. [Google Scholar] [CrossRef]
- Xu, L.Q.; Yang, W.J.; Neoh, K.-G.; Kang, E.-T.; Fu, G.D. Dopamine-Induced Reduction and Functionalization of Graphene Oxide Nanosheets. Macromolecules 2010, 43, 8336–8339. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Qiblawey, H.; Khraisheh, M. Polydopamine Functionalized Graphene Oxide as Membrane Nanofiller: Spectral and Structural Studies. Membranes 2021, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Jayabal, E.; Natarajan, A.; Rengarajan, V. One-Pot Hydrothermal Synthesis of Nitrogen-Doped Reduced Graphene Oxide for the Highly Sensitive and Simultaneous Determination of Dihydroxy Benzene Isomers. J. Appl. Electrochem. 2021, 51, 1189–1205. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of Graphene-Based Nanosheets via Chemical Reduction of Exfoliated Graphite Oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, S.; Fang, Y.; Dong, S. Reducing Sugar: New Functional Molecules for the Green Synthesis of Graphene Nanosheets. ACS Nano 2010, 4, 2429–2437. [Google Scholar] [CrossRef]
- Alotaibi, F.; Tung, T.T.; Nine, M.J.; Kabiri, S.; Moussa, M.; Tran, D.N.H.; Losic, D. Scanning Atmospheric Plasma for Ultrafast Reduction of Graphene Oxide and Fabrication of Highly Conductive Graphene Films and Patterns. Carbon 2018, 127, 113–121. [Google Scholar] [CrossRef]
- Chen, L.I.N.; Perfetti, C.A.; Leng, Y. Reading Pinyin Activates Character Orthography for Highly Experienced Learners of Chinese. Biling. Lang. Cogn. 2017, 22, 103–111. [Google Scholar] [CrossRef]
- Zhou, Y.; Bao, Q.; Tang, L.A.L.; Zhong, Y.; Loh, K.P. Hydrothermal Dehydration for the “Green” Reduction of Exfoliated Graphene Oxide to Graphene and Demonstration of Tunable Optical Limiting Properties. Chem. Mater. 2009, 21, 2950–2956. [Google Scholar] [CrossRef]
- Fan, X.; Cai, C.; Gao, J.; Han, X.; Li, J. Hydrothermal Reduced Graphene Oxide Membranes for Dyes Removing. Sep. Purif. Technol. 2020, 241, 116730. [Google Scholar] [CrossRef]
- Toh, S.Y.; Loh, K.S.; Kamarudin, S.K.; Daud, W.R.W. Graphene Production via Electrochemical Reduction of Graphene Oxide: Synthesis and Characterisation. Chem. Eng. J. 2014, 251, 422–434. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Qiblawey, H. Synergetic Effects of Dodecylamine-Functionalized Graphene Oxide Nanoparticles on Antifouling and Antibacterial Properties of Polysulfone Ultrafiltration Membranes. J. Water Process Eng. 2021, 42, 102120. [Google Scholar] [CrossRef]
- Liu, Y.; Gan, D.; Chen, M.; Ma, L.; Yang, B.; Li, L.; Zhu, M.; Tu, W. Bioinspired Dopamine Modulating Graphene Oxide Nanocomposite Membrane Interposed by Super-Hydrophilic UiO-66 with Enhanced Water Permeability. Sep. Purif. Technol. 2020, 253, 117552. [Google Scholar] [CrossRef]
- Lu, K.-J.; Zuo, J.; Chung, T.-S. Novel PVDF Membranes Comprising N-Butylamine Functionalized Graphene Oxide for Direct Contact Membrane Distillation. J. Membr. Sci. 2017, 539, 34–42. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhu, Y.; Liu, X.; Zhu, P.; Hu, Y.; Sun, R.; Wong, C.-P. A Flexible Pressure Sensor Based on Melamine Foam Capped by Copper Nanowires and Reduced Graphene Oxide. Mater. Today Commun. 2020, 24, 100970. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Wang, L.; Zhao, B.; Zhang, Z.; Xia, X.; Yang, H.; Xue, Y.; Chang, N. Enhancement of Hydrophilicity and the Resistance for Irreversible Fouling of Polysulfone (PSF) Membrane Immobilized with Graphene Oxide (GO) through Chloromethylated and Quaternized Reaction. Chem. Eng. J. 2018, 334, 2068–2078. [Google Scholar] [CrossRef]
- Malek Alaie, M.; Jahangiri, M.; Rashidi, A.M.; Haghighi Asl, A.; Izadi, N. A Novel Selective H2S Sensor Using Dodecylamine and Ethylenediamine Functionalized Graphene Oxide. J. Ind. Eng. Chem. 2015, 29, 97–103. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, L.; Feng, L.; Lu, H.; Xu, Y.; Wang, J.; Xiang, B.; Zou, X. Polydopamine Functionalized Graphene Oxide Nanocomposites Reinforced the Corrosion Protection and Adhesion Properties of Waterborne Polyurethane Coatings. Eur. Polym. J. 2019, 120, 109249. [Google Scholar] [CrossRef]
- Arshad, F.; Selvaraj, M.; Zain, J.; Banat, F.; Haija, M.A. Polyethylenimine Modified Graphene Oxide Hydrogel Composite as an Efficient Adsorbent for Heavy Metal Ions. Sep. Purif. Technol. 2019, 209, 870–880. [Google Scholar] [CrossRef]
- Han, Z.; Tang, Z.; Sun, Y.; Yang, J.; Zhi, L. Controllable Synthesis of Tetraethylenepentamine Modified Graphene Foam (TEPA-GF) for the Removal of Lead Ions OPEN. Nat. Publ. Group 2015, 5, 16730. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sajjadi, B.; Chen, W.-Y.; Chatterjee, R. Ultrasound-Assisted Amine Functionalized Graphene Oxide for Enhanced CO2 Adsorption. Fuel 2019, 247, 10–18. [Google Scholar] [CrossRef]
- Ribeiro, H.; da Silva, W.M.; Neves, J.C.; Calado, H.D.R.; Paniago, R.; Seara, L.M.; das Mercês Camarano, D.; Silva, G.G. Multifunctional Nanocomposites Based on Tetraethylenepentamine-Modified Graphene Oxide/Epoxy. Polym. Test. 2015, 43, 182–192. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Qiblawey, H.; Khraisheh, M.; Atieh, M.; Al-Ghouti, M. Synthesis of Graphene Oxides Particle of High Oxidation Degree Using a Modified Hummers Method. Ceram. Int. 2020, 46, 23997–24007. [Google Scholar] [CrossRef]
- Guerrero-Contreras, J.; Caballero-Briones, F. Graphene Oxide Powders with Different Oxidation Degree, Prepared by Synthesis Variations of the Hummers Method. Mater. Chem. Phys. 2015, 153, 209–220. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Deng, T.; Li, F. Reclamation of EDTA by Sodium Tetraethylenepentamine-Multi Dithiocarbamate after Soil Washing Process with EDTA. Environ. Earth Sci. 2017, 76, 311. [Google Scholar] [CrossRef]
- Long, Q.W.; Wang, Y. Sodium Tetraethylenepentamine Heptaacetate as Novel Draw Solute for Forward Osmosis—Synthesis, Application and Recovery. Energies 2015, 8, 12917–12928. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Zhao, H.; Zhang, B.; Fang, P. Stabilization of Cadmium- and Lead-Contaminated Sites Using Sodium Tetraethylenepentamine-Multi Dithiocarbamate. Water Air Soil Pollut. 2016, 228, 6. [Google Scholar] [CrossRef]
- López-Díaz, D.; López Holgado, M.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman Spectroscopy in Graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and Structural Studies of High Quality Graphene Oxide and Reduced Graphene Oxide Prepared by Different Chemical Oxidation Methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Navaee, A.; Salimi, A. Efficient Amine Functionalization of Graphene Oxide through the Bucherer Reaction: An Extraordinary Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. RSC Adv. 2015, 5, 59874–59880. [Google Scholar] [CrossRef]
- Ni, Z.H.; Wang, H.M.; Kasim, J.; Fan, H.M.; Yu, T.; Wu, Y.H.; Feng, Y.P.; Shen, Z.X. Graphene Thickness Determination Using Reflection and Contrast Spectroscopy. Nano Lett. 2007, 7, 2758–2763. [Google Scholar] [CrossRef] [PubMed]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-Area Ultrathin Films of Reduced Graphene Oxide as a Transparent and Flexible Electronic Material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef]
- Ajdari, F.B.; Kowsari, E.; Ehsani, A.; Chepyga, L.; Schirowski, M.; Jäger, S.; Kasian, O.; Hauke, F.; Ameri, T. Melamine-Functionalized Graphene Oxide: Synthesis, Characterization and Considering as Pseudocapacitor Electrode Material with Intermixed POAP Polymer. Appl. Surf. Sci. 2018, 459, 874–883. [Google Scholar] [CrossRef]
- Saini, P.; Singh, M.; Singh, S.P.; Mahapatro, A.K. Spectroscopic and Electronic Properties of Polyallylamine Functionalized Graphene Oxide Films. Vacuum 2018, 154, 110–114. [Google Scholar] [CrossRef]
- Sengupta, J.; Das, K.; Nandi, U.N.; Jacob, C. Substrate Free Synthesis of Graphene Nanoflakes by Atmospheric Pressure Chemical Vapour Deposition Using Ni Powder as a Catalyst. Bull. Mater. Sci. 2019, 42, 136. [Google Scholar] [CrossRef] [Green Version]
- Rabchinskii, M.K.; Dideikin, A.T.; Kirilenko, D.A.; Baidakova, M.V.; Shnitov, V.V.; Roth, F.; Konyakhin, S.V.; Besedina, N.A.; Pavlov, S.I.; Kuricyn, R.A.; et al. Facile Reduction of Graphene Oxide Suspensions and Films Using Glass Wafers. Sci. Rep. 2018, 8, 14154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Song, Y.; Dai, K.; Sun, S.; Liu, G.; Yao, J. Novel Ternary Nanohybrids of Tetraethylenepentamine and Graphene Oxide Decorated with MnFe2O4 Magnetic Nanoparticles for the Adsorption of Pb(II). J. Hazard. Mater. 2018, 358, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Lochab, B. A Comparative Study of Graphene Oxide: Hummers, Intermediate and Improved Method. FlatChem 2019, 13, 40–49. [Google Scholar] [CrossRef]
- Talyzin, A.V.; Mercier, G.; Klechikov, A.; Hedenström, M.; Johnels, D.; Wei, D.; Cotton, D.; Opitz, A.; Moons, E. Brodie vs. Hummers Graphite Oxides for Preparation of Multi-Layered Materials. Carbon 2017, 115, 430–440. [Google Scholar] [CrossRef] [Green Version]












| Sample | Peak | Binding Energy (eV) | at.% | C/O (at) |
|---|---|---|---|---|
| GO | C 1s | 284 | 77.79 | 3.53 |
| O 1s | 530 | 22.06 | ||
| S 2p | 168 | 0.15 | ||
| rGO-TEPA1 | C 1s | 285 | 77.88 | 4.43 |
| O 1s | 532 | 17.60 | ||
| N 1s | 400 | 4.36 | ||
| S 2p | 169 | 0.16 | ||
| rGO-TEPA2 | C 1s | 285 | 78.21 | 5.59 |
| O 1s | 532 | 13.98 | ||
| N 1s | 400 | 7.73 | ||
| S 2p | 169 | 0.08 |
| Sample | N (wt.%) | C (wt.%) | H (wt.%) | O (wt.%) | C/O (wt%) |
|---|---|---|---|---|---|
| GO | 0 | 57.02 | 1.99 | 40.99 | 1.4 |
| rGO-TEPA1 | 4.60 | 63.31 | 2.82 | 29.27 | 2.2 |
| rGO-TEPA2 | 6.20 | 66.20 | 3.14 | 24.46 | 2.7 |
| GO:TEPA | Catalytic/Reducing Agent | Method and Conditions | C/O | Reduction % * | Ref. |
|---|---|---|---|---|---|
| 1:100 | Hydrazine hydrate | Hydrothermal reduction (180 °C, 5 h) | 1.6 a | - | [7] |
| 1:5 | - | Hydrothermal reduction (180 °C, 20 h) | - | - | [23] |
| 1:10 | EDC and HOBT | Ultrasound-assisted and chemical activation (40 °C, 24 h) | 3.59 b | 7.6 b | [24] |
| 1:1500 | - | Microwave radiation-assisted reaction (120 °C, 30 min) | - | - | [25] |
| 1:10 | - | Temperature-assisted solution interaction (60 °C, 48 h) | 5.59 a 2.7 b | 36.6 a 40.3 b | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhouzaam, A.; Abdelrazeq, H.; Khraisheh, M.; AlMomani, F.; Hameed, B.H.; Hassan, M.K.; Al-Ghouti, M.A.; Selvaraj, R. Spectral and Structural Properties of High-Quality Reduced Graphene Oxide Produced via a Simple Approach Using Tetraethylenepentamine. Nanomaterials 2022, 12, 1240. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12081240
Alkhouzaam A, Abdelrazeq H, Khraisheh M, AlMomani F, Hameed BH, Hassan MK, Al-Ghouti MA, Selvaraj R. Spectral and Structural Properties of High-Quality Reduced Graphene Oxide Produced via a Simple Approach Using Tetraethylenepentamine. Nanomaterials. 2022; 12(8):1240. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12081240
Chicago/Turabian StyleAlkhouzaam, Abedalkader, Haneen Abdelrazeq, Majeda Khraisheh, Fares AlMomani, Bassim H. Hameed, Mohammad K. Hassan, Mohammad A. Al-Ghouti, and Rengaraj Selvaraj. 2022. "Spectral and Structural Properties of High-Quality Reduced Graphene Oxide Produced via a Simple Approach Using Tetraethylenepentamine" Nanomaterials 12, no. 8: 1240. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12081240