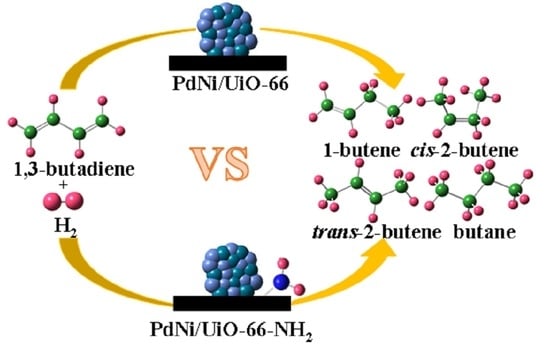
Comparative Study of Pd–Ni Bimetallic Catalysts Supported on UiO-66 and UiO-66-NH2 in Selective 1,3-Butadiene Hydrogenation
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of UiO-66-NH2 and UiO-66
2.2. Synthesis of Pd–Ni Bimetallic Catalysts
2.3. Catalytic Activity Measurement
3. Results and Discussion
3.1. Characterization of Catalysts
3.2. Catalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Q.; Hou, R.; Sun, K. Tuning butene selectivities by Cu modification on Pd-based catalyst for the selective hydrogenation of 1,3-butadiene. J. Catal. 2019, 374, 12–23. [Google Scholar] [CrossRef]
- Yi, H.; Du, H.; Hu, Y.; Yan, H.; Jiang, H.L.; Lu, L. Precisely controlled porous alumina overcoating on Pd catalyst by atomic layer deposition: Enhanced selectivity and durability in hydrogenation of 1,3-butadiene. ACS Catal. 2015, 5, 2735–2739. [Google Scholar] [CrossRef]
- Yang, Q.; Sun, K.; Xu, Y.; Ding, Z.; Hou, R. Tuning crystal phase of molybdenum carbide catalyst to induce the different selective hydrogenation performance. Appl. Catal. A Gen. 2022, 630, 118455–118465. [Google Scholar] [CrossRef]
- Yardimci, D.; Serna, P.; Gates, B.C. A highly selective catalyst for partial hydrogenation of 1,3-butadiene: MgO-supported rhodium clusters selectively poisoned with CO. ChemCatChem 2012, 4, 1547–1550. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Z.X. Alloying effect on the C–C coupling reactions in acetylene hydrogenation by palladium-coinage metal alloys, a DFT study and microkinetic modeling. Appl. Surf. Sci. 2022, 575, 151513–151521. [Google Scholar] [CrossRef]
- Pattamakomsan, K.; Ehret, E.; Morfin, F.; Gélin, P.; Jugnet, Y.; Prakash, S.; Bertolini, J.C.; Panpranot, J.; Aires, F.J.C.S. Selective hydrogenation of 1,3-butadiene over Pd and Pd–Sn catalysts supported on different phases of alumina. Catal. Today 2011, 164, 28–33. [Google Scholar] [CrossRef]
- Odoom-Wubah, T.; Li, Q.; Chen, M.; Fang, H.; Bediako, B.B.A.; Adilov, I.; Huang, J.; Li, Q. Influence of preparation methods on the catalytic activity of Pd−Cu/Mn2O3 catalyst in the hydrogenation of 1,3-butadiene. ACS Omega 2019, 4, 1300–1310. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Yang, B. Theoretical understandings on the unusual selectivity of 1,3-butadiene hydrogenation to butenes over gold catalysts. Catal. Today 2020, 347, 134–141. [Google Scholar] [CrossRef]
- Hu, C.; Shao, M.; Xiang, M.; Li, S.; Xu, S. The role of hydrogen coverage and location in 1,3-butadiene hydrogenation over Pt/SiO2. React. Chem. Eng. 2020, 5, 87–100. [Google Scholar] [CrossRef]
- Hu, N.; Li, X.Y.; Liu, S.M.; Wang, Z.; He, X.K.; Hou, Y.X.; Wang, Y.X.; Deng, Z.; Chen, L.H.; Su, B.L. Enhanced stability of highly-dispersed copper catalyst supported by hierarchically porous carbon for long term selective hydrogenation. Chin. J. Catal. 2020, 41, 1081–1090. [Google Scholar] [CrossRef]
- He, X.; Li, X.; Wang, Z.; Hu, N.; Deng, Z.; Chen, L.; Su, B. Self-reduction for the synthesis of Co supported on hierarchically porous carbon for selective hydrogenation reaction. Chem. J. Chin. Univ. Chin. 2020, 41, 639–645. [Google Scholar]
- Feng, Y.; Zhou, L.; Wan, Q.; Lin, S.; Guo, H. Selective hydrogenation of 1,3-butadiene catalyzed by a single Pd atom anchored on graphene: The importance of dynamics. Chem. Sci. 2018, 9, 5890–5896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachiller-Baeza, B.; Iglesias-Juez, A.; Agostini, G.; Castillejos-López, E. Pd–Au bimetallic catalysts supported on ZnO for selective 1,3-butadiene hydrogenation. Catal. Sci. Technol. 2020, 10, 2503–2512. [Google Scholar] [CrossRef]
- Hoeven, J.V.D.; Jelic, J.; Olthof, L.A.; Totarella, G.; Dijk-Moes, R.J.A.V.; Krafft, J.M.; Louis, C.; Studt, F.; Blaaderen, A.V.; Jongh, P.E.D. Unlocking synergy in bimetallic catalysts by core-shell design. Nat. Mater. 2021, 20, 1216–1220. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Sun, D.; Jiang, X. Plant-mediated synthesis of AgPd/γ-Al2O3 catalysts for selective hydrogenation of 1,3-butadiene at low temperature. New J. Chem. 2019, 43, 13891–13898. [Google Scholar] [CrossRef]
- Ma, H.; Xu, X.; Xu, H.; Feng, H.; Yuan, X.; Cheng, D. Understanding composition-dependent catalytic performance of PdAg for the hydrogenation of 1,3-butadiene to 1-butene. Catal. Common. 2021, 149, 106255–106260. [Google Scholar] [CrossRef]
- Méndez, F.J.; Piccolo, L.; Solano, R.; Aouine, M.; Villasana, Y.; Guerra, J.; Curbelo, S.; Olivera-Fuentes, C.; Brito, J. Promoting effect of ceria on the performance of NiPd/CeO2-Al2O3 catalysts for the selective hydrogenation of 1,3-butadiene in the presence of 1-butene. New J. Chem. 2018, 42, 11165–11173. [Google Scholar] [CrossRef]
- Aguilar-Tapia, A.; Delannoy, L.; Louis, C.; Han, C.W.; Ortalan, V.; Zanella, R. Selective hydrogenation of 1,3-butadiene over bimetallic Au-Ni/TiO2 catalysts prepared by deposition-precipitation with urea. J. Catal. 2016, 344, 515–523. [Google Scholar] [CrossRef] [Green Version]
- Lucci, F.R.; Liu, J.; Marcinkowski, M.D.; Yang, M.; Allard, L.F.; Flytzani–Stephanopoulos, M.; Sykes, E.C.H. Selective hydrogenation of 1,3-butadiene on platinum-copper alloys at the single-atom limit. Nat. Commun. 2015, 6, 8550–8557. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Yan, H.; Huang, L.; Zhang, X.; Lin, Y.; Li, J.; Xia, Y.; Ma, Y.; Sun, Z.; Wei, S.; et al. Toward understanding of the support effect on Pd1 single-atom-catalyzed hydrogenation reactions. J. Phys. Chem. C 2019, 123, 7922–7930. [Google Scholar] [CrossRef]
- Hou, R.; Porosoff, M.D.; Chen, J.G.; Wang, T. Effect of oxide supports on Pd–Ni bimetallic catalysts for 1,3-butadiene hydrogenation. Appl. Catal. A Gen. 2015, 490, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Decarolis, D.; Lezcano-Gonzalez, I.; Gianolio, D.; Beale, A.M. Effect of particle size and support type on Pd catalysts for 1,3-butadiene hydrogenation. Top. Catal. 2018, 61, 162–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonergan, W.W.; Wang, T.; Vlachos, D.G.; Chen, J.G. Effect of oxide support surface area on hydrogenation activity: Pt/Ni bimetallic catalysts supported on low and high surface area Al2O3 and ZrO2. Appl. Catal. A Gen. 2011, 408, 87–95. [Google Scholar] [CrossRef]
- Wang, T.; Mpourmpakis, G.; Lonergan, W.W.; Vlachos, D.G.; Chen, J.G. Effect of oxide supports in stabilizing desirable Pt–Ni bimetallic structures for hydrogenation and reforming reactions. Phys. Chem. Chem. Phys. 2013, 15, 12156–12164. [Google Scholar] [CrossRef]
- Selvakannan, P.R.; Hoang, L.; Kumar, V.V.; Dumbre, D.; Jampaiah, D.; Das, J.; Bhargava, S.K. Selective hydrogenation of 1,3-butadiene to 1-butene: Review on catalysts, selectivity, kinetics and reaction mechanism. Catal. Clean Energy Environ. Sustain. 2021, 495, 205–228. [Google Scholar]
- Lonergan, W.W.; Vlachos, D.G.; Chen, J.G. Correlating extent of Pt–Ni bond formation with low-temperature hydrogenation of benzene and 1,3-butadiene over supported Pt/Ni bimetallic catalysts. J. Catal. 2010, 271, 239–250. [Google Scholar] [CrossRef]
- Cai, F.; Yang, L.; Shan, S.; Mott, D.; Chen, B.; Luo, J.; Zhong, C.J. Preparation of PdCu alloy nanocatalysts for nitrate hydrogenation and carbon monoxide oxidation. Catalysts 2016, 6, 96. [Google Scholar] [CrossRef] [Green Version]
- Insorn, P.; Kitiyanan, B. Selective hydrogenation of concentrated vinyl acetylene mixed C4 by modified Pd catalysts: Effect of Cu. Catalysts 2016, 6, 199. [Google Scholar] [CrossRef] [Green Version]
- Méndez, F.J.; Solano, R.; Villasana, Y.; Guerra, J.; Curbelo, S.; Inojosa, M.; Olivera-Fuentes, C. Selective hydrogenation of 1,3-butadiene in presence of 1-butene under liquid phase conditions with NiPd/Al2O3 catalysts. Appl. Petrochem. Res. 2016, 6, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, G.; Louis, C.; Delannoy, L. Novel non-noble bimetallic Cu-Zn/TiO2 catalysts for selective hydrogenation of butadiene. J. Catal. 2017, 347, 185–196. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, X.; Guo, L.; Yan, S.; Li, Y.; Jiang, S.; Tai, X. Bimetallic Au–Pd alloy nanoparticles supported on MIL-101(Cr) as highly efficient catalysts for selective hydrogenation of 1,3-butadiene. RSC Adv. 2020, 10, 33417–33427. [Google Scholar] [CrossRef]
- Singh, B.K.; Lee, S.; Na, K. An overview on metal-related catalysts: Metal oxides, nanoporous metals and supported metal nanoparticles on metal organic frameworks and zeolites. Rare Met. 2020, 39, 751–766. [Google Scholar] [CrossRef]
- Wei, Z.H.; Zhang, R.R.; Mu, L.N.; Huang, Y.P.; Liu, Z.S. Fabrication of core-shell sol-gel hybrid molecularly imprinted polymer based on metal–organic framework. Eur. Polym. J. 2019, 121, 109301–109308. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, W.D.; Zhao, C.F.; Yan, J. Dimensional reduction of Eu-based metal-organic framework as catalysts for oxidation catalysis of C(sp3)–H bond. Chin. J. Chem. 2022, 40, 480–486. [Google Scholar] [CrossRef]
- Vakili, R.; Slater, T.J.A.; Hardacre, C.; Walton, A.S.; Fan, X. PtNi bimetallic structure supported on UiO-67 metal-organic framework (MOF) during CO oxidation. J. Catal. 2020, 391, 522–529. [Google Scholar] [CrossRef]
- Liu, P.; Gu, X.; Wu, Y.; Cheng, J.; Su, H. Construction of bimetallic nanoparticles immobilized by porous functionalized metal-organic frameworks toward remarkably enhanced catalytic activity for the room-temperature complete conversion of hydrous hydrazine into hydrogen. Int. J. Hydrogen Energy 2017, 42, 19096–19105. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, X.; Yan, Y.; Zhou, J.; Zhang, W.; Tai, X. Bimetallic gold-silver nanoparticles supported on zeolitic imidazolate framework-8 as highly active heterogeneous catalysts for selective oxidation of benzyl alcohol into benzaldehyde. Polymers 2018, 10, 1089. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.H.; Yu, J.; Wei, Z.C.; Guo, X.M.; Mao, H.F.; Mao, D.S. Preparation and characterization of UiO-66-supported Cu-Ce bimetal catalysts for low-temperature CO oxidation. Catal. Lett. 2019, 149, 496–506. [Google Scholar] [CrossRef]
- Ten, S.; Torbina, V.V.; Zaikovskii, V.I.; Kulinich, S.A.; Vodyankina, O.V. Bimetallic AgPd/UiO-66 hybrid catalysts for propylene glycol oxidation into lactic acid. Materials 2020, 13, 5471. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, B.; Chai, X.; Liu, J.; Gu, J.; Ning, P. Comparison of Pd-UiO-66 and Pd-UiO-66-NH2 catalysts performance for phenol hydrogenation in aqueous medium. Fuel 2017, 205, 130–141. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, C.; Caro, J.; Huang, A. A new UiO-66-NH2 based mixed-matrix membranes with high CO2/CH4 separation performance. Microporous Mesoporous Mater. 2019, 274, 203–211. [Google Scholar] [CrossRef]
- Liu, R.; Meng, S.; Ma, Y.; Niu, L.; He, S.; Xu, X.; Su, B.; Lu, D.; Yang, Z.; Lei, Z. Atmospherical oxidative coupling of amines by UiO-66-NH2 photocatalysis under milder reaction conditions. Catal. Commun. 2019, 124, 108–112. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.H.; Rutledge, W.; Li, J.R.; Zhou, H.C. Zr-based metal-organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Liu, J.; Xiong, Z.; Zhang, Z.; Guan, Z. Selectivity adsorptive mechanism of different nitrophenols on UIO-66 and UIO-66-NH2 in aqueous solution. J. Chem. Eng. Data 2016, 61, 3868–3876. [Google Scholar] [CrossRef]
- Cseri, L.; Hardian, R.; Anan, S.; Vovusha, H.; Schwingenschlögl, U.; Budd, P.M.; Sada, K.; Kokado, K.; Szekely, G. Bridging the interfacial gap in mixed-matrix membranes by nature-inspired design: Precise molecular sieving with polymer-grafted metal–organic frameworks. J. Mater. Chem. A 2021, 9, 23793–23802. [Google Scholar] [CrossRef]
- Zhu, H.; Huang, J.; Zhou, Q.; Lv, Z.; Li, C.; Hu, G. Enhanced luminescence of NH2-UiO-66 for selectively sensing fluoride anion in water medium. J. Lumin. 2019, 208, 67–74. [Google Scholar] [CrossRef]
- Kirakci, K.; Bůžek, D.; Peer, P.; Liška, V.; Mosinger, J.; Křížová, I.; Kloda, M.; Ondrušová, S.; Lang, K.; Demel, J. Polymeric membranes containing iodine-loaded UiO-66 nanoparticles as water-responsive antibacterial and antiviral surfaces. ACS Appl. Nano Mater. 2022, 5, 1244–1251. [Google Scholar] [CrossRef]
- Guo, Z.; Xiao, C.; Maligal-Ganesh, R.V.; Zhou, L.; Goh, T.W.; Li, X.; Tesfagaber, D.; Thiel, A.; Huang, W. Pt nanoclusters confined within metal-organic framework cavities for chemoselective cinnamaldehyde hydrogenation. ACS Catal. 2014, 4, 1340–1348. [Google Scholar] [CrossRef]
- Yoshimaru, S.; Sadakiyo, M.; Maeda, N.; Yamauchi, M.; Kato, K.; Pirillo, J.; Hijikata, Y. Support effect of metal-organic frameworks on ethanol production through acetic acid hydrogenation. ACS Appl. Mater. Interfaces 2021, 13, 19992–20001. [Google Scholar] [CrossRef]
- Stawowowy, M.; Ciesielski, R.; Maniecki, T.; Matus, K.; Łużny, R.; Trawczynski, J.; Silvestre-Albero, J.; Łamacz, A. CO2 hydrogenation to methanol over Ce and Zr containing UiO-66 and Cu/UiO-66. Catalysts 2020, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Primet, M.; El Azhar, M.; Guenin, M. Influence of the support towards platinum catalysed 1,3-butadiene hydrogenation. Appl. Catal. 1990, 58, 241–253. [Google Scholar] [CrossRef]
- Chakarova, K.; Strauss, I.; Mihaylov, M.; Drenchev, N.; Hadjiivanov, K. Evolution of acid and basic sites in UiO-66 and UiO-66-NH2 metal-organic frameworks: FTIR study by probe molecules. Microporous Mesoporous Mater. 2019, 281, 110–122. [Google Scholar] [CrossRef]
- Wang, G.N.; Chen, L.M.; Guo, Y.Y.; Fu, M.L.; Wu, J.L.; Huang, B.C.; Ye, D.Q. Effect of chromium doping on the catalytic behavior of Cu/ZrO2/CNTs-NH2 for the synthesis of methanol from carbon dioxide hydrogenation. Acta Phys. Chim. Sin. 2014, 30, 923–931. [Google Scholar]
- Xu, L.; Wang, Q.; Liang, D.; Wang, X.; Lin, L.; Cui, W.; Xu, Y. The promotions of MnO and K2O to Fe/silicalite-2 catalyst for the production of light alkenes from CO2 hydrogenation. Appl. Catal. A Gen. 1998, 173, 19–25. [Google Scholar] [CrossRef]
- Zheng, X.X.; Shen, L.J.; Chen, X.P.; Zheng, X.H.; Au, C.T.; Jiang, L.L. Amino-modified Fe-terephthalate metal−organic framework as an efficient catalyst for the selective oxidation of H2S. Inorg. Chem. 2018, 57, 10081–10089. [Google Scholar] [CrossRef]
- Ewald, S.; Hinrichsen, O. On the interaction of CO2 with Ni-Al catalysts. Appl. Catal. A Gen. 2019, 580, 71–80. [Google Scholar] [CrossRef]
- Liu, L.L.; Zhou, X.J.; Liu, L.; Jiang, S.; Li, Y.J.; Guo, L.X.; Yan, S.J.; Tai, X.S. Heterogeneous bimetallic Cu–Ni nanoparticle-supported catalysts in the selective oxidation of benzyl alcohol to benzaldehyde. Catalysts 2019, 9, 538. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhang, X.; Rang, S.; Yang, Y.; Dai, X.; Gao, J.; Xu, C.; He, J. Catalysis by metal–organic frameworks: Proline and gold functionalized MOFs for the aldol and three-component coupling reactions. RSC Adv. 2014, 4, 13093–13107. [Google Scholar]
- Liu, L.; Tai, X.; Zhou, X.; Liu, L.; Zhang, X.; Ding, L.; Zhang, Y. Au-Pt bimetallic nanoparticle catalysts supported on UiO-67 for selective 1,3-butadiene hydrogenation. J. Taiwan Inst. Chem. Eng. 2020, 114, 220–227. [Google Scholar] [CrossRef]
- Liu, L.L.; Tai, X.S.; Zhou, X.J.; Hou, J.X.; Zhang, Z.H. Bimetallic Au-Ni alloy nanoparticles in a metal-organic framework (MIL-101) as efficient heterogeneous catalysts for selective oxidation of benzyl alcohol into benzaldehyde. J. Alloys Compd. 2019, 790, 326–336. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, H.; Zhao, X.; Cao, T.; Chen, J.; Zhu, W.; Yu, Y.; Hou, Z. Au–Pd nanoparticles on layered double hydroxide: Highly active catalyst for aerobic oxidation of alcohols in aqueous phase. Catal. Commun. 2012, 18, 142–146. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, M.; Zhang, Z.; Liu, C.; Xu, S.; Li, Z. Zinc phthalocyanine coupled with UIO-66 (NH2) via a facile condensation process for enhanced visible-light-driven photocatalysis. J. Alloys Compd. 2017, 690, 123–130. [Google Scholar] [CrossRef]
- Zhang, K.; Kong, T.; Zeng, Y.; Tan, Z. Effect of support modified by a alkaline additive on the properties of Pd/Al2O3-TiO2 catalysts. Pet. Process. Petrochem. 2011, 42, 38–42. [Google Scholar]
- Hou, R.; Yu, W.; Porosoff, M.D.; Chen, J.G.; Wang, T. Selective hydrogenation of 1,3-butadiene on PdNi bimetallic catalyst: From model surfaces to supported catalysts. J. Catal. 2014, 316, 1–10. [Google Scholar] [CrossRef]










| Entry | Sample | BET (m2/g) | Mean Pore Diameter (nm) | Volume (cm3/g) |
|---|---|---|---|---|
| 1 | UiO-66 | 1467 | 2.1 | 0.75 |
| 2 | PdNi/UiO-66 (1:1) | 1292 | 2.2 | 0.70 |
| 3 | UiO-66-NH2 | 1223 | 3.3 | 1.02 |
| 4 | PdNi/UiO-66-NH2 (1:1) | 851 | 3.8 | 0.81 |
| Entry | Catalyst | Mean Particle Size (nm) | T (°C) | Conv. (%) | Sel. (%) | Lifetime (h) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | PdNi/UiO-66 (1:1) | 7.1 | 40 | 99.8 | 84.5 | 9 | This work |
| 2 | PdNi/UiO-66-NH2 (1:1) | 4.6 | 60 | 100 | 89.5 | 16 | This work |
| 3 | PdNi/γ-Al2O3 | 5.9 | 70 | 90 | 80 | - | 21 |
| 4 | PdNi/SiO2 | 6.3 | 70 | 85 | 100 | - | 21 |
| 5 | 1NiPd/Al2O3 | - | 40 | 80 | 99.8 | - | 29 |
| 6 | 0.91%Pd1.51%Ni/γ-Al2O3 | 5.9 | 87 | 100 | - | - | 64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Yu, L.; Zhou, X.; Xin, C.; Sun, S.; Liu, Z.; Zhang, J.; Liu, Y.; Tai, X. Comparative Study of Pd–Ni Bimetallic Catalysts Supported on UiO-66 and UiO-66-NH2 in Selective 1,3-Butadiene Hydrogenation. Nanomaterials 2022, 12, 1484. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12091484
Liu L, Yu L, Zhou X, Xin C, Sun S, Liu Z, Zhang J, Liu Y, Tai X. Comparative Study of Pd–Ni Bimetallic Catalysts Supported on UiO-66 and UiO-66-NH2 in Selective 1,3-Butadiene Hydrogenation. Nanomaterials. 2022; 12(9):1484. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12091484
Chicago/Turabian StyleLiu, Lili, Lei Yu, Xiaojing Zhou, Chunling Xin, Songyuan Sun, Zhidong Liu, Jinyu Zhang, Ying Liu, and Xishi Tai. 2022. "Comparative Study of Pd–Ni Bimetallic Catalysts Supported on UiO-66 and UiO-66-NH2 in Selective 1,3-Butadiene Hydrogenation" Nanomaterials 12, no. 9: 1484. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12091484