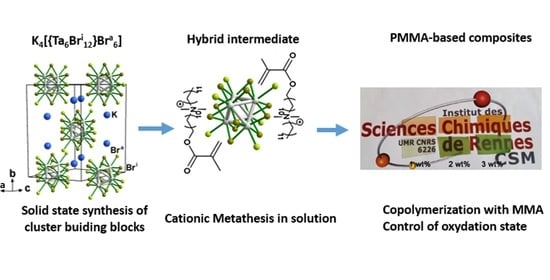
From Solid-State Cluster Compounds to Functional PMMA-Based Composites with UV and NIR Blocking Properties, and Tuned Hues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. Synthesis of the (Kat)2[{Ta6Bri12}Bra6] Intermediate by Metathesis Reaction
- 1H-NMR (400 MHz, CDCl3) δ ppm: 6.11 (d, 2H, CHH=C), 5.57 (d, 2H, CHH=C), 4.15 (t, 4H, –CH2–O), 3.51 (br, 8H, –CH2–N+), 3.38 (s, 6H, CH3–N+), 1.94 (s, 6H,–CH3–C), 1.7–1.67 (m, 8H, –(CH2CH2)2N, 1.66 (m, 4H, –CH2–CH2–O), 1.33–1.26 (m, 64H, –CH2), 0.86 (t, 6H, –CH3).
- d: doublet, t: triplet, br: broad signal, s: singlet, m: multiplet.
- EDAX: no potassium Ta 27%, Br 73% (Theo: 25%, Br 75%).
2.1.2. Polymerization with Methyl Methacrylate
2.1.3. Ta_x@PMMAbrown Composites Film Deposition
2.1.4. Reduction of Ta_x@PMMAbrown Polymer Composites
2.2. Instrumentation
2.2.1. NMR Experiments
2.2.2. Energy Dispersive Scattering (EDS)
2.2.3. Infrared (IR) Spectroscopy
2.2.4. Thermal Analysis
2.2.5. UV-Vis-NIR Experiments
3. Results
3.1. Synthesis of Functional Ta6 Cluster Precursors and Ta_x@PMMA Composites
3.2. Modulation of Oxidation States of Ta6 Cluster Building Blocks in PMMA Matrix and Characterization of Thin Films
3.3. Optical Properties of Ta6@PMMA Nanocomposite Films in the Oxidized and Reduced Form
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanu, S.S.; Binions, R. Thin films for solar control applications. Proc. Math. Phys. Eng. Sci. 2010, 466, 19–44. [Google Scholar] [CrossRef] [Green Version]
- Soumya, S.; Mohamed, A.P.; Paul, L.; Mohan, K.; Ananthakumar, S. Near IR reflectance characteristics of PMMA/ZnO nanocomposites for solar thermal control interface films. Sol. Energy Mater. Sol. Cells 2014, 125, 102–112. [Google Scholar] [CrossRef]
- Li, S.; Toprak, M.S.; Jo, Y.S.; Dobson, J.; Kim, D.K.; Muhammed, M. Bulk Synthesis of Transparent and Homogeneous Polymeric Hybrid Materials with ZnO Quantum Dots and PMMA. Adv. Mater. 2007, 19, 4347–4352. [Google Scholar] [CrossRef]
- Reyes-Acosta, M.A.; Torres-Huerta, A.M.; Domínguez-Crespo, M.A.; Flores-Vela, A.I.; Dorantes-Rosales, H.J.; Andraca-Adame, J.A. Thermal, Mechanical and UV-Shielding Properties of Poly(Methyl Methacrylate)/Cerium Dioxide Hybrid Systems Obtained by Melt Compounding. Polymers 2015, 7, 1638–1659. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhuang, S.; Xu, X.; Hu, J. Transparent and UV-shielding ZnO@PMMA nanocomposite films. Opt. Mater. 2013, 36, 169–172. [Google Scholar] [CrossRef]
- Rezaei, S.D.; Shannigrahi, S.; Ramakrishna, S. A review of conventional, advanced, and smart glazing technologies and materials for improving indoor environment. Sol. Energy Mater. Sol. Cells 2017, 159, 26–51. [Google Scholar] [CrossRef]
- Rosati, A.; Fedel, M.; Rossi, S. NIR reflective pigments for cool roof applications: A comprehensive review. J. Clean. Prod. 2021, 313, 127826. [Google Scholar] [CrossRef]
- Levinson, R.; Berdahl, P.; Akbari, H. Solar spectral optical properties of pigments—Part II: Survey of common colorants. Sol. Energy Mater. Sol. Cells 2005, 89, 351–389. [Google Scholar] [CrossRef]
- Gao, Q.; Wu, X.; Huang, T. Greatly improved NIR shielding performance of CuS nanocrystals by gallium doping for energy efficient window. Ceram. Int. 2021, 47, 23827–23833. [Google Scholar] [CrossRef]
- Gao, Q.; Wu, X.; Huang, T. Novel energy efficient window coatings based on In doped CuS nanocrystals with enhanced NIR shielding performance. Sol. Energy 2021, 220, 1–7. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Li, J.; Wang, K.; Zhang, G. Z-scheme g-C3N4@CsxWO3 heterostructure as smart window coating for UV isolating, Vis penetrating, NIR shielding and full spectrum photocatalytic decomposing VOCs. Appl. Catal. B Environ. 2018, 229, 218–226. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Q.; Shi, F.; Liu, S.; Luo, J.; Bao, L.; Feng, X. Dispersion of Cs0.33WO3 particles for preparing its coatings with higher near infrared shielding properties. Appl. Surf. Sci. 2014, 309, 175–180. [Google Scholar] [CrossRef]
- Taallah, H.; Chorfa, A.; Tamayo, A.; Rubio, F.; Rubio, J. Investigating the effect of WO3 on the crystallization behavior of SiO2–B2O3–Al2O3–Na2O–CaO–ZnO high VIS-NIR reflecting glazes. Ceram. Int. 2021, 47, 26789–26799. [Google Scholar] [CrossRef]
- Tan, W.K.; Yokoi, A.; Kawamura, G.; Matsuda, A.; Muto, H. PMMA-ITO Composite Formation via Electrostatic Assembly Method for Infra-Red Filtering. Nanomaterials 2019, 9, 886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Chen, Z.; Wei, W.; Zhang, P.; Zhu, Y.; Zhao, Q.; Lv, K.; Liu, X.; Gao, Y. Cs0.32WO3/PMMA nanocomposite via in-situ polymerization for energy saving windows. Sol. Energy Mater. Sol. Cells 2020, 215, 110656. [Google Scholar] [CrossRef]
- Lebastard, C.; Wilmet, M.; Cordier, S.; Comby-Zerbino, C.; MacAleese, L.; Dugourd, P.; Hara, T.; Ohashi, N.; Uchikoshi, T.; Grasset, F. Controlling the Deposition Process of Nanoarchitectonic Nanocomposites Based on {Nb6−xTaxXi12}n+ Octahedral Cluster-Based Building Blocks (Xi = Cl, Br; 0 ≤ x ≤ 6, n = 2, 3, 4) for UV-NIR Blockers Coating Applications. Nanomaterials 2022, 12, 2052. [Google Scholar] [CrossRef] [PubMed]
- Lebastard, C.; Wilmet, M.; Cordier, S.; Comby-Zerbino, C.; MacAleese, L.; Dugourd, P.; Ohashi, N.; Uchikoshi, T.; Grasset, F. High performance {Nb5TaX12}@PVP (X = Cl, Br) cluster-based nanocomposites coatings for solar glazing applications. Sci. Technol. Adv. Mater. 2022, 23, 446–456. [Google Scholar] [CrossRef]
- Cotton, F.A. Metal Atom Clusters in Oxide Systems. Inorg. Chem. 1964, 3, 1217–1220. [Google Scholar] [CrossRef]
- Costuas, K.; Garreau, A.; Bulou, A.; Fontaine, B.; Cuny, J.; Gautier, R.; Mortier, M.; Molard, Y.; Duvail, J.L.; Faulques, E.; et al. Combined theoretical and time-resolved photoluminescence investigations of [Mo6Bri8Bra6]2− metal cluster units: Evidence of dual emission. Phys. Chem. Chem. Phys. 2015, 17, 28574–28585. [Google Scholar] [CrossRef]
- Dierre, B.; Costuas, K.; Dumait, N.; Paofai, S.; Amela-Cortes, M.; Molard, Y.; Grasset, F.; Cho, Y.; Takahashi, K.; Ohashi, N.; et al. Mo6 cluster-based compounds for energy conversion applications: Comparative study of photoluminescence and cathodoluminescence. Sci. Technol. Adv. Mater. 2017, 18, 458–466. [Google Scholar] [CrossRef]
- Aubert, T.; Grasset, F.; Mornet, S.; Duguet, E.; Cador, O.; Cordier, S.; Molard, Y.; Demange, V.; Mortier, M.; Haneda, H. Functional silica nanoparticles synthesized by water-in-oil microemulsion processes. J. Colloid Interf. Sci. 2010, 341, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Feliz, M.; Puche, M.; Atienzar, P.; Concepción, P.; Cordier, S.; Molard, Y. In Situ Generation of Active Molybdenum Octahedral Clusters for Photocatalytic Hydrogen Production from Water. ChemSusChem 2016, 9, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Guy, K.; Ehni, P.; Paofai, S.; Forschner, R.; Roiland, C.; Amela-Cortes, M.; Cordier, S.; Laschat, S.; Molard, Y. Lord of The Crowns: A New Precious in the Kingdom of Clustomesogens. Angew. Chem. Int. Ed. 2018, 57, 11692–11696. [Google Scholar] [CrossRef]
- Kepenekian, M.; Molard, Y.; Costuas, K.; Lemoine, P.; Gautier, R.; Ababou Girard, S.; Fabre, B.; Turban, P.; Cordier, S. Red-NIR luminescence of Mo6 monolayered assembly directly anchored on Au(001). Mater. Horiz. 2019, 6, 1828–1833. [Google Scholar] [CrossRef]
- Molard, Y.; Dorson, F.; Cîrcu, V.; Roisnel, T.; Artzner, F.; Cordier, S. Clustomesogens: Liquid Crystal Materials Containing Transition-Metal Clusters. Angew. Chem. Int. Ed. 2010, 49, 3351–3355. [Google Scholar] [CrossRef] [PubMed]
- Prévôt, M.; Amela-Cortes, M.; Manna, S.K.; Cordier, S.; Roisnel, T.; Folliot, H.; Dupont, L.; Molard, Y. Electroswitchable red-NIR luminescence of ionic clustomesogen containing nematic liquid crystalline devices. J. Mater. Chem. C 2015, 3, 5152–5161. [Google Scholar] [CrossRef]
- Renaud, A.; Grasset, F.; Dierre, B.; Uchikoshi, T.; Ohashi, N.; Takei, T.; Planchat, A.; Cario, L.; Jobic, S.; Odobel, F.; et al. Inorganic Molybdenum Clusters as Light-Harvester in All Inorganic Solar Cells: A Proof of Concept. ChemistrySelect 2016, 1, 2284–2289. [Google Scholar] [CrossRef]
- Wilmet, M.; Lebastard, C.; Sciortino, F.; Comby-Zerbino, C.; MacAleese, L.; Chirot, F.; Dugourd, P.; Grasset, F.; Matsushita, Y.; Uchikoshi, T.; et al. Revisiting properties of edge-bridged bromide tantalum clusters in the solid-state, in solution and vice versa: An intertwined experimental and modelling approach. Dalton Trans. 2021, 50, 8002–8016. [Google Scholar] [CrossRef]
- Hernández, J.S.; Shamshurin, M.; Puche, M.; Sokolov, M.N.; Feliz, M. Nanostructured Hybrids Based on Tantalum Bromide Octahedral Clusters and Graphene Oxide for Photocatalytic Hydrogen Evolution. Nanomaterials 2022, 20, 3647. [Google Scholar] [CrossRef]
- Cooke, N.E.; Kuwana, T.; Espenson, J.H. Electrochemistry of tantalum bromide cluster compound. Inorg. Chem. 1971, 10, 1081–1083. [Google Scholar] [CrossRef]
- Espenson, J.H.; Boone, D.J. Kinetics and mechanism of oxidation of the tantalum halide cluster ion (Ta6Cl12)2+ by cobalt(III) complexes and by miscellaneous oxidizing agents. Inorg. Chem. 1968, 7, 636–640. [Google Scholar] [CrossRef]
- Espenson, J.H.; Kinney, R.J. Kinetic study of the oxidation of the tantalum cluster ion Ta6Br122+ by chromium(VI). Inorg. Chem. 1971, 10, 376–378. [Google Scholar] [CrossRef]
- Kuhn, P.J.; McCarley, R.E. Chemistry of Polynuclear Metal Halides. I. Preparation of the Polynuclear Tantalum Halides Ta6X14. Inorg. Chem. 1965, 4, 1482–1486. [Google Scholar] [CrossRef]
- Prokopuk, N.; Kennedy, V.O.; Stern, C.L.; Shriver, D.F. Substitution and Redox Chemistry of [Bu4N]2[Ta6Cl12(OSO2CF3)6]. Inorg. Chem. 1998, 37, 5001–5006. [Google Scholar] [CrossRef]
- Chabrie, M.C. Sur un nouveau chlorure de tantale. Compt. Rend. 1907, 144, 804–806. [Google Scholar]
- Chapin, W.H. Halide Bases of Tantalum. J. Am. Chem. Soc. 1910, 32, 323–330. [Google Scholar] [CrossRef]
- Harned, H.S. Halide Bases of Columbium. J. Am. Chem. Soc. 1913, 35, 1078–1086. [Google Scholar] [CrossRef]
- Cramer, P.; Bushnell, D.A.; Fu, J.; Gnatt, A.L.; Maier-Davis, B.; Thompson, N.E.; Burgess, R.R.; Edwards, A.M.; David, P.R.; Kornberg, R.D. Architecture of RNA polymerase II and implications for the transcription mechanism. Science 2000, 288, 640–649. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, K.N.; Iverson, T.M.; Maghlaoui, K.; Barber, J.; Iwata, S. Architecture of the photosynthetic oxygen-evolving center. Science 2004, 303, 1831–1838. [Google Scholar] [CrossRef] [Green Version]
- Kamiguchi, S.; Nagashima, S.; Chihara, T. Characterization of Catalytically Active Octahedral Metal Halide Cluster Complexes. Metals 2014, 4, 84–107. [Google Scholar] [CrossRef] [Green Version]
- Lowe, J.; Stock, D.; Jap, B.; Zwickl, P.; Baumeister, W.; Huber, R. Crystal structure of the 20S proteasome from the archaeon T. acidophilum at 3.4 A resolution. Science 1995, 268, 533. [Google Scholar] [CrossRef] [PubMed]
- Mullan, B.F.; Madsen, M.T.; Messerle, L.; Kolesnichenko, V.; Kruger, J. X-ray attenuation coefficients of high-atomic-number, hexanuclear transition metal cluster compounds: A new paradigm for radiographic contrast agents. Acad. Radiol. 2000, 7, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Sokolov Maxim, N.; Abramov Pavel, A.; Mikhailov Maxim, A.; Peresypkina Eugenia, V.; Virovets Alexander, V.; Fedin Vladimir, P. Simplified Synthesis and Structural Study of {Ta6Br12} Clusters. Z. Anorg. Allg. Chem. 2010, 636, 1543–1548. [Google Scholar] [CrossRef]
- Amela-Cortes, M.; Garreau, A.; Cordier, S.; Faulques, E.; Duvail, J.-L.; Molard, Y. Deep red luminescent hybrid copolymer materials with high transition metal cluster content. J. Mater. Chem. C 2014, 2, 1545–1552. [Google Scholar] [CrossRef] [Green Version]
- Amela-Cortes, M.; Molard, Y.; Paofai, S.; Desert, A.; Duvail, J.-L.; Naumov, N.G.; Cordier, S. Versatility of the ionic assembling method to design highly luminescent PMMA nanocomposites containing [M6Qi8La6]n− octahedral nano-building blocks. Dalton Trans. 2016, 45, 237–245. [Google Scholar] [CrossRef]
- Li, H.; Qi, W.; Li, W.; Sun, H.; Bu, W.; Wu, L. A Highly Transparent and Luminescent Hybrid Based on the Copolymerization of Surfactant-Encapsulated Polyoxometalate and Methyl Methacrylate. Adv. Mater. 2005, 17, 2688–2692. [Google Scholar] [CrossRef]
- Nguyen, T.K.N.; Renaud, A.; Wilmet, M.; Dumait, N.; Paofai, S.; Dierre, B.; Chen, W.; Ohashi, N.; Cordier, S.; Grasset, F.; et al. New ultra-violet and near-infrared blocking filters for energy saving applications: Fabrication of tantalum metal atom cluster-based nanocomposite thin films by electrophoretic deposition. J. Mater. Chem. C 2017, 5, 10477–10484. [Google Scholar] [CrossRef]
- Renaud, A.; Wilmet, M.; Truong, T.G.; Seze, M.; Lemoine, P.; Dumait, N.; Chen, W.; Saito, N.; Ohsawa, T.; Uchikoshi, T.; et al. Transparent tantalum cluster-based UV and IR blocking electrochromic devices. J. Mater. Chem. C 2017, 5, 8160–8168. [Google Scholar] [CrossRef]
- Espenson, J.H.; McCarley, R.E. Oxidation of Tantalum Cluster Ions1. J. Am. Chem. Soc. 1966, 88, 1063–1064. [Google Scholar] [CrossRef]
- McCarley, R.E.; Hughes, B.G.; Cotton, F.A.; Zimmerman, R. The Two-Electron Oxidation of Metal Atom Cluster Species of the Type [M6X12]2+. Inorg. Chem. 1965, 4, 1491–1492. [Google Scholar] [CrossRef]
- Spreckelmeyer, B.; Schäfer, H. Die photometrische Titration des [Ta6Br12]2+-Ions. J. Less Common Met. 1967, 13, 127–129. [Google Scholar] [CrossRef]
- Rudine, A.B.; Walter, M.G.; Wamser, C.C. Reaction of Dichloromethane with Pyridine Derivatives under Ambient Conditions. J. Org. Chem. 2010, 75, 4292–4295. [Google Scholar] [CrossRef] [PubMed]
- Cook, D. Vibrational Spectra of Pyridium Salts. Can. J. Chem. 1961, 39, 2009–2024. [Google Scholar] [CrossRef]
- Koleva, B.B.; Kolev, T.; Seidel, R.W.; Tsanev, T.; Mayer-Figge, H.; Spiteller, M.; Sheldrick, W.S. Spectroscopic and structural elucidation of 4-dimethylaminopyridine and its hydrogensquarate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008, 71, 695–702. [Google Scholar] [CrossRef]
- Fleming, P.B.; Meyer, J.L.; Grindstaff, W.K.; McCarley, R.E. Chemistry of polynuclear metal halides. VIII. Infrared spectra of some Nb6X12n+ and Ta6X12n+ derivatives. Inorg. Chem. 1970, 9, 1769–1771. [Google Scholar] [CrossRef]
- Harder, K.; Preetz, W. Schwingungsspektren der Clusterverbindungen (M6X)X · 8 H2O, M = Nb, Ta; Xi = CI, Br; Xa = CI, Br, I. Z. Anorg. Allg. Chem. 1990, 591, 32–40. [Google Scholar] [CrossRef]
- Mackay, R.A.; Schneider, R.F. Far-infrared spectra of compounds containing the M6X12 metal atom cluster. Inorg. Chem. 1968, 7, 455–459. [Google Scholar] [CrossRef]
- Rufino, E.S.; Monteiro, E.E.C. Infrared study on methyl methacrylate–methacrylic acid copolymers and their sodium salts. Polymer 2003, 44, 7189–7198. [Google Scholar] [CrossRef]
- Stokes, N.L.; Edgar, J.A.; McDonagh, A.M.; Cortie, M.B. Spectrally selective coatings of gold nanorods on architectural glass. J. Nanoparticle Res. 2010, 12, 2821–2830. [Google Scholar] [CrossRef] [Green Version]
- Carboni, M.; Carravetta, M.; Zhang, X.L.; Stulz, E. Efficient NIR light blockage with matrix embedded silver nanoprism thin films for energy saving window coating. J. Mater. Chem. C 2016, 4, 1584–1588. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.T.K.; Lebastard, C.; Wilmet, M.; Dumait, N.; Renaud, A.; Cordier, S.; Ohashi, N.; Uchikoshi, T.; Grasset, F. A review on functional nanoarchitectonics nanocomposites based on octahedral metal atom clusters (Nb6, Mo6, Ta6, W6, Re6): Inorganic 0D and 2D powders and films. Sci. Technol. Adv. Mater. 2022, 23, 547–578. [Google Scholar] [CrossRef] [PubMed]
- Soumya, S.; Sheemol, V.N.; Amba, P.; Mohamed, A.P.; Ananthakumar, S. Sn and Ag doped ZnO quantum dots with PMMA by in situ polymerization for UV/IR protective, photochromic multifunctional hybrid coatings. Sol. Energy Mater. Sol. Cells 2018, 174, 554–565. [Google Scholar] [CrossRef]
- Mitsuoka, T.; Torikai, A.; Fueki, K. Wavelength sensitivity of the photodegradation of poly(methyl methacrylate). J. Appl. Polym. Sci. 1993, 47, 1027–1032. [Google Scholar] [CrossRef]
- Torikai, A.; Ohno, M.; Fueki, K. Photodegradation of poly(methyl methacrylate) by monochromatic light: Quantum yield, effect of wavelengths, and light intensity. J. Appl. Polym. Sci. 1990, 41, 1023–1032. [Google Scholar] [CrossRef]
- Koziej, D.; Fischer, F.; Kränzlin, N.; Caseri, W.R.; Niederberger, M. Nonaqueous TiO2 Nanoparticle Synthesis: A Versatile Basis for the Fabrication of Self-Supporting, Transparent, and UV-Absorbing Composite Films. ACS Appl. Mater. Interfaces 2009, 1, 1097–1104. [Google Scholar] [CrossRef]
- Singhal, A.; Dubey, K.A.; Bhardwaj, Y.K.; Jain, D.; Choudhury, S.; Tyagi, A.K. UV-shielding transparent PMMA/In2O3 nanocomposite films based on In2O3 nanoparticles. RSC Adv. 2013, 3, 20913–20921. [Google Scholar] [CrossRef]
- Schelm, S.; Smith, G.B.; Garrett, P.D.; Fisher, W.K. Tuning the surface-plasmon resonance in nanoparticles for glazing applications. J. Appl. Phys. 2005, 97, 124314. [Google Scholar] [CrossRef]
- Roy, A.; Ghosh, A.; Mallick, T.K.; Tahir, A.A. Smart glazing thermal comfort improvement through near-infrared shielding paraffin incorporated SnO2-Al2O3 composite. Constr. Build. Mater. 2022, 331, 127319. [Google Scholar] [CrossRef]
- Ding, C.; Han, A.; Ye, M.; Zhang, Y.; Yao, L.; Yang, J. Synthesis and characterization of a series of new green solar heat-reflective pigments: Cr-doped BiPO4 and its effect on the aging resistance of PMMA (Poly(methyl methacrylate)). Sol. Energy Mater. Sol. Cells 2019, 191, 427–436. [Google Scholar] [CrossRef]
- Zhou, W.; Ye, J.; Liu, Z.; Wang, L.; Chen, L.; Zhuo, S.; Liu, Y.; Chen, W. High Near-Infrared Reflective Zn1–xAxWO4 Pigments with Various Hues Facilely Fabricated by Tuning Doped Transition Metal Ions (A = Co, Mn, and Fe). Inorg. Chem. 2022, 61, 693–699. [Google Scholar] [CrossRef]




| wt% Ta6 Cluster | Mass Cluster/mg | mmol Cluster | Mass DMAP/mg | mmol DMAP |
|---|---|---|---|---|
| 1 | 10 | 0.003 | 0.006 | 0.71 |
| 2 | 20 | 0.006 | 0.12 | 1.46 |
| 3 | 30 | 0.009 | 0.18 | 2.20 |
| Sample | Tg (°C) | Tvis | Tsol | Tvis/sol |
|---|---|---|---|---|
| PMMA | 114 | |||
| Ta6_1@PMMAbrown | 85 | 81.2 | 80.8 | 1.00 |
| Ta6_2@PMMAbrown | 87 | 65.3 | 70.6 | 0.92 |
| Ta6_3@PMMAbrown | 89 | 55.2 | 64.8 | 0.85 |
| Ta6_1@PMMAgreen | 79 | 81.0 | 77.4 | 1.05 |
| Ta6_2@PMMAgreen | 93 | 50.6 | 53.1 | 0.95 |
| Ta6_3@PMMAgreen | 97 | 43.3 | 49.0 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amela-Cortes, M.; Wilmet, M.; Le Person, S.; Khlifi, S.; Lebastard, C.; Molard, Y.; Cordier, S. From Solid-State Cluster Compounds to Functional PMMA-Based Composites with UV and NIR Blocking Properties, and Tuned Hues. Nanomaterials 2023, 13, 144. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13010144
Amela-Cortes M, Wilmet M, Le Person S, Khlifi S, Lebastard C, Molard Y, Cordier S. From Solid-State Cluster Compounds to Functional PMMA-Based Composites with UV and NIR Blocking Properties, and Tuned Hues. Nanomaterials. 2023; 13(1):144. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13010144
Chicago/Turabian StyleAmela-Cortes, Maria, Maxence Wilmet, Samuel Le Person, Soumaya Khlifi, Clément Lebastard, Yann Molard, and Stéphane Cordier. 2023. "From Solid-State Cluster Compounds to Functional PMMA-Based Composites with UV and NIR Blocking Properties, and Tuned Hues" Nanomaterials 13, no. 1: 144. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13010144