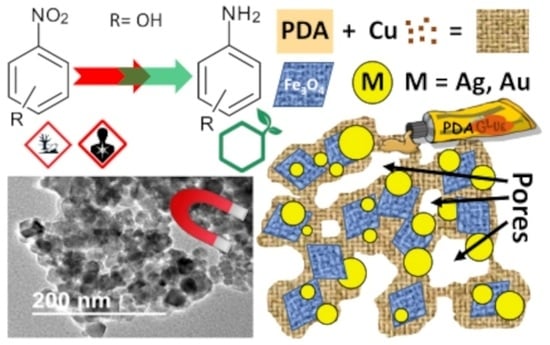
Synthesis and Catalytic Activity for 2, 3, and 4-Nitrophenol Reduction of Green Catalysts Based on Cu, Ag and Au Nanoparticles Deposited on Polydopamine-Magnetite Porous Supports
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Techniques
- SEM (Scanning Electron Microscopy) microstructural characterizations were carried out using a JEOL JEM-1010 instrument with a CCD camera operating at 100 kV. This instrument was also used to determine the metal contents by energy dispersive X-ray spectroscopy (EDX) analysis.
- The TEM, STEM-HAADF, and mapping of different elements (by using an EDS X-ray detector) was carried out using a JEOL-2100F microscope operated at 200 kV. For electron microscopy analyses, the samples were dispersed in ethanol and placed onto a carbon-coated nickel microgrid and left to dry before observation.
- TGA (Thermal Gravimetric Analysis) curves were recorded using a Setaram Setsys 16/18 thermobalance with a heating rate of 5 C/min under an air flowing atmosphere of 25 mL/min.
- XRD (Powder X-ray Diffraction) was carried out using a Bruker D8 Advance diffractometer with monochromatic K source operated at 40 kV and 40 mA. Patterns were collected in steps of 0.02(2) over the angular range 1–10(2) with an acquisition time of 25 s per step.
- Nitrogen adsorption–desorption isotherms were recorded in an automated Micromeritics ASAP2020 instrument. Prior to the adsorption measurements, the samples were outgassed in situ under vacuum ( Torr) at 120 C for 15 h to remove adsorbed gases.
- DLS (Dynamic Light Scattering) photonic correlation diagrams used to determine hydrodynamic radii were measured using a Zetasizer nano series instrument (from Malvern Instruments).
- Absorbance was monitored to track reaction progress using a diode-array UV/Vis Agilent 8453 spectrometer.
- HPLC mixture resolutions were carried out using a JASCO HPLC modular chromatograph equipped with a C18 polar column (Kinetex, 100 mm × 4.6 mm, 2.6 m particle size from Phenomenex) fitted to a column guard, a quaternary pump (PU-2089+), column oven (CO-2065+), and a diode-array detector (MD-2018+).
2.3. Preparation of M NPs-PDA@Fe3O4 Catalysts (M = Cu, Ag, Au, Ag/Cu)
2.3.1. Synthesis of Fe3O4
2.3.2. Coating Fe3O4 with Polydopamine (PDA@Fe3O4, Thin and Thick Films)
2.3.3. Synthesis of Ag NPs-PDA@Fe3O4 Catalysts (Thick Film)
- Catalyst C1 was prepared by adding dropwise a solution of ascorbic acid (112 mg, 0.63 mmol) in water (20 mL) to a suspension of Ag(I)-PDA@Fe3O4 (200 mg) in water (10 mL), and left to stir in the dark for 24 h.
- Catalyst C2 was obtained by stirring a suspension of Ag(I) NPs-PDA@Fe3O4 (200 mg) in water (20 mL) in sunlight for 24 h.
- Catalyst C3 was prepared by adding dropwise a solution of NaBH4 (80 mg, 2.11 mmol) in water (5 mL) to an aqueous suspension of Ag(I)-PDA@Fe3O4 (200 mg, 20 mL of water), and left to stir in the dark for 24 h.
2.3.4. Synthesis of Au NPs-PDA@Fe3O4 Catalysts (Thick Film)
- Catalyst C4 was obtained by slowly adding a solution of ascorbic acid (17.1 mg, 0.10 mmol) in water (20 mL) via a perfusion pump (0.5 mL/min) to the Au(III) NPs-PDA@Fe3O4 dispersion. This mixture was heated under stirring at 65 C for 60 min.
- Catalyst C5 was obtained only by stirring the Au(III) NPs-PDA@Fe3O4 in water (20 mL) dispersion whilst heating at 75 C for 1 h.
- Catalyst C6 was prepared by slowly adding a solution of NaBH4 (10.9 mg, 0.29 mmol) in water (5 mL) via a perfusion pump to the Au(III) NPs-PDA@Fe3O4 dispersion, and left to stir at 65 C for 60 min.
2.3.5. Synthesis of Cu NPs-PDA@Fe3O4 Catalyst (C7, Thin Film)
2.3.6. Synthesis of Ag NPs-PDA@Fe3O4 Catalyst (C8, Thin Film)
2.3.7. Synthesis of Ag/Cu NPs-PDA@Fe3O4 Catalyst (C9, Thin Film)
2.4. Procedure for the Study of Nitrophenol Reduction with UV/Vis Spectroscopy
2.5. Catalyst Recyclability
3. Results and Discussion
3.1. Synthesis of MNPs-PDA@Fe3O4 Catalysts (M = Ag, Au)
3.2. Characterization of MNPs-PDA@Fe3O4 Catalysts
3.2.1. Thermogravimetric Analysis
3.2.2. X-ray Diffraction Results
3.2.3. Scanning Electron Microscopy
3.2.4. Transmission Electron Microscopy and STEM
3.2.5. N2 Absorption/Desorption Isotherms
3.2.6. Dynamic Light Scattering
3.3. Study of Catalyst Activity
Evaluation of Activity
3.4. Catalyst Recyclability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.; Somerville, R.J.; Chen, L.; Yu, Y.; Fei, Z.; Wang, S.; Dyson, P.J.; Min, D. Carbonized wood impregnated with bimetallic nanoparticles as a monolithic continuous-flow microreactor for the reduction of 4-nitrophenol. J. Hazard. Mater. 2023, 443, 130270. [Google Scholar] [CrossRef] [PubMed]
- Bakhsh, E.M.; Ismail, M.; Sharafat, U.; Akhtar, K.; Fagieh, T.M.; Danish, E.Y.; Khan, S.B.; Khan, M.I.; Khan, M.A.; Asiri, A.M. Highly efficient and recoverable Ag-Cu bimetallic catalyst supported on taro-rhizome powder applied for nitroarenes and dyes reduction. J. Mater. Res. Technol. 2022, 18, 769–787. [Google Scholar] [CrossRef]
- Sachi; Singh, A.P.; Thirumal, M. Fabrication of AgNi nano-alloy-decorated ZnO nanocomposites as an efficient and novel hybrid catalyst to degrade noxious organic pollutants. ACS Omega 2021, 6, 34771–34782. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Huang, C.; Zheng, Z.; Zhong, B.; Ding, G.; Li, J.; You, L.; Wang, S. Preparation of magnetically recoverable MPCTP-Ag composite nanoparticles and their application as high-performance catalysts. Langmuir 2021, 37, 10249–10258. [Google Scholar] [CrossRef]
- Chishti, A.N.; Ma, Z.; Liu, Y.; Chen, M.; Gautam, J.; Guo, F.; Ni, L.; Diao, G. Synthesis of highly efficient and magnetically separable Fe3O4@C-TiO2-Ag catalyst for the reduction of organic dyes and 4-nitrophenol. Colloids Surf. A 2021, 631, 127694. [Google Scholar] [CrossRef]
- Chishti, A.N.; Guo, F.; Aftab, A.; Ma, Z.; Liu, Y.; Chen, M.; Gautam, J.; Chen, C.; Ni, L.; Diao, G. Synthesis of silver doped Fe3O4/C nanoparticles and its catalytic activities for the degradation and reduction of methylene blue and 4-nitrophenol. Appl. Surf. Sci. 2021, 546, 149070. [Google Scholar] [CrossRef]
- Fagieh, T.M.; Bakhsh, E.M.; Khan, S.B.; Akhtar, K.; Asiri, A.M. Alginate/Banana Waste Beads Supported Metal Nanoparticles for Efficient Water Remediation. Polymers 2021, 13, 4054. [Google Scholar] [CrossRef]
- Benmaati, A.; Boukoussa, B.; Aoul, R.H.; Hachemaoui, M.; Kerbadou, R.M.; Zahmani, H.H.; Hacini, S. Insights into Catalytic Reduction of Organic Pollutants Catalyzed by Nanoparticles Supported on Zeolite Clinoptilolite. Silicon 2022, 14, 8831–8843. [Google Scholar] [CrossRef]
- Gondwal, M.; Sharma, N.; nee Pant, G.J.; Gautam, B.P.S.; Singh, S.; Tumba, K.; Bahadur, I. Bioactivity and Catalytic Reduction of Aryl Nitro-Compounds by Biosynthesized Silver Nanoparticles using Skimmiaanquetilia. ChemistrySelect 2023, 8, e202203782. [Google Scholar] [CrossRef]
- Erdem, H.B.; Çetinkaya, S. Facile insitu preparation of silver nanoparticles supported on petroleum asphaltene-derived porous carbon for efficient reduction of nitrophenols. Heliyon 2022, 8, e10659. [Google Scholar] [CrossRef]
- Sánchez, E.G.; Melero, M.M.; Picot, M.Á.Ú.; Pérez-Pla, F.; Marcos, M.D.; Haskouri, J.E.; Amorós, P. Nitroarene hydrogenation catalysts based on Pd nanoparticles glued with PDA on inorganic supports: Multivariate Curve Resolution as an useful tool to compare the catalytic activity in multi-step reactions. Appl. Catal. A Gen. 2021, 619, 118125. [Google Scholar] [CrossRef]
- Chishti, A.N.; Ni, L.; Guo, F.; Lin, X.; Liu, Y.; Wu, H.; Chen, M.; Diao, G.W. Magnetite-Silica core-shell nanocomposites decorated with silver nanoparticles for enhanced catalytic reduction of 4-nitrophenol and degradation of methylene blue dye in the water. J. Environ. Chem. Eng. 2021, 9, 104948. [Google Scholar] [CrossRef]
- Wei, Z.; Feng, D.; Li, J.; Lin, Y.; Zhang, H. Nanosheet array-like Cu@Cu2O-CuNiAl(O)/rGO composites for highly efficient reduction of nitrophenol: Electronic and structure promotion effect of nickel. Chem. Eng. J. 2022, 427, 131659. [Google Scholar] [CrossRef]
- Xie, S.; Xu, Z.; Yu, C.; Yu, X.; Zhang, Z.; Li, J. Highly Efficient Reduction of 4-Nitrophenol by Cu Nanoparticle Decorated Graphdiyne. ChemistrySelect 2021, 6, 13572–13576. [Google Scholar] [CrossRef]
- Mejía, Y.R.; Bogireddy, N.K.R. Reduction of 4-nitrophenol using green-fabricated metal nanoparticles. RSC Adv. 2022, 12, 18661–18675. [Google Scholar] [CrossRef]
- Das, T.K.; Das, N.C. Advances on catalytic reduction of 4-nitrophenol by nanostructured materials as benchmark reaction. Int. Nano Lett. 2022, 12, 223–242. [Google Scholar] [CrossRef]
- Formenti, D.; Ferretti, F.; Scharnagl, F.K.; Beller, M. Reduction of nitro compounds using 3d-non-noble metal catalysts. Chem. Rev. 2019, 119, 2611–2680. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Akbari, R.; Sakhaei, S.; Nezafat, Z.; Banazadeh, S.; Orooji, Y.; Hegde, G. Polymer supported copper complexes/nanoparticles for treatment of environmental contaminants. J. Mol. Liq. 2021, 330, 115668. [Google Scholar] [CrossRef]
- Kottappara, R.; Pillai, S.C.; Vijayan, B.K. Copper-based nanocatalysts for nitroarene reduction—A review of recent advances. Inorg. Chem. Commun. 2020, 121, 108181. [Google Scholar] [CrossRef]
- Orlandi, M.; Brenna, D.; Harms, R.; Jost, S.; Benaglia, M. Recent developments in the reduction of aromatic and aliphatic nitro compounds to amines. Org. Process Res. Dev. 2018, 22, 430–445. [Google Scholar] [CrossRef]
- Lara, L.R.S.; Zottis, A.D.; Elias, W.C.; Faggion, D.; Maduro de Campos, C.E.; Acuña, J.J.S.; Domingos, J.B. The catalytic evaluation of in situ grown Pd nanoparticles on the surface of Fe3O4@dextran particles in the p-nitrophenol reduction reaction. RSC Adv. 2015, 5, 8289–8296. [Google Scholar] [CrossRef]
- Begum, R.; Rehan, R.; Farooqi, Z.H.; Butt, Z.; Ashraf, S. Physical chemistry of catalytic reduction of nitroarenes using various nanocatalytic systems: Past, present, and future. J. Nanoparticle Res. Interdiscip. Forum Nanoscale Sci. Technol. 2016, 18, 231. [Google Scholar] [CrossRef]
- Kadam, H.K.; Tilve, S.G. Advancement in methodologies for reduction of nitroarenes. RSC Adv. 2015, 5, 83391–83407. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic concepts and recent advances in nitrophenol reduction by gold- and other transition metal nanoparticles. Coord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Aditya, T.; Pal, A.; Pal, T. Nitroarene reduction: A trusted model reaction to test nanoparticle catalysts. Chem. Commun. 2015, 51, 9410–9431. [Google Scholar] [CrossRef]
- Lara, P.; Philippot, K. The hydrogenation of nitroarenes mediated by platinum nanoparticles: An overview. Catal. Sci. Technol. 2014, 4, 2445–2465. [Google Scholar] [CrossRef]
- Malik, A.; Nath, M. Synthesis of Ag/ZIF-7 by immobilization of Ag nanoparticles onto ZIF-7 microcrystals: A heterogeneous catalyst for the reduction of nitroaromatic compounds and organic dyes. J. Environ. Chem. Eng. 2020, 8, 104547. [Google Scholar] [CrossRef]
- Qi, Y.; Ye, J.; Ren, S.; Lv, J.; Zhang, S.; Che, Y.; Ning, G. In-situ synthesis of metal nanoparticles@metal-organic frameworks: Highly effective catalytic performance and synergistic antimicrobial activity. J. Hazard. Mater. 2020, 387, 121687. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine surface chemistry: A decade of discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Liu, Y.; Han, Y.; Chen, R.; Zhang, H.; Liu, S.; Liang, F. In situ Immobilization of Copper Nanoparticles on Polydopamine Coated Graphene Oxide for H2O2 Determination. PLoS ONE 2016, 11, e0157926. [Google Scholar] [CrossRef] [Green Version]
- Beckford, S.; Mathurin, L.; Chen, J.; Fleming, R.A.; Zou, M. The effects of polydopamine coated Cu nanoparticles on the tribological properties of polydopamine/PTFE coatings. Tribol. Int. 2016, 103, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Li, D.; Yang, H.; Guo, X. Fabrication of Fe3O4@polydopamine@polyamidoamine core shell nanocomposites and their application for Cu(scp/scp) adsorption. New J. Chem. 2018, 42, 12212–12221. [Google Scholar] [CrossRef]
- Hemmati, S.; Zangeneh, M.M.; Zangeneh, A. CuCl2 anchored on polydopamine coated-magnetic nanoparticles (Fe3O4@PDA/Cu(II)): Preparation, characterization and evaluation of its cytotoxicity, antioxidant, antibacterial, and antifungal properties. Polyhedron 2020, 177, 114327. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Wang, J.; Yu, D.; Li, Z.; Liu, R. Facile synthesis of silver nanocatalyst decorated Fe3O4@PDA core–shell nanoparticles with enhanced catalytic properties and selectivity. RSC Adv. 2022, 12, 3847–3855. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, H.; Wang, B.; Kong, Y.; Chen, J. Silver-incorporated mussel-inspired polydopamine coatings on mesoporous silica as an efficient nanocatalyst and antimicrobial agent. ACS Appl. Mater. Interfaces 2018, 10, 1792–1801. [Google Scholar] [CrossRef]
- Das, T.K.; Ganguly, S.; Remanan, S.; Ghosh, S.; Das, N.C. Mussel-inspired Ag/poly(norepinephrine)/MnO2 heterogeneous nanocatalyst for efficient reduction of 4-nitrophenol and 4-nitroaniline: An alternative approach. Res. Chem. Intermed. 2020, 46, 3629–3650. [Google Scholar] [CrossRef]
- Duan, J.; Bai, L.; Xu, K.; Fang, Q.; Sun, Y.; Xu, H.; Leung, K.C.F.; Xuan, S. Polydopamine protected hollow nanosphere with AuAg-nanoframe-core@Carbon@AuAg-nanocrystals-satellite hybrid nanostructure (AuAg@C@AuAg/PDA) for enhancing nanocatalysis. J. Hazard. Mater. 2020, 384, 121276. [Google Scholar] [CrossRef]
- Erdoğan, H. Catalytic degradation of 4-Nitrophenol and methylene blue by bioinspired polydopamine coated dipeptide structures. Colloid Interface Sci. Commun. 2020, 39, 100331. [Google Scholar] [CrossRef]
- Cheng, D.; Zhang, Y.; Liu, Y.; Bai, X.; Ran, J.; Bi, S.; Deng, Z.; Tang, X.; Wu, J.; Cai, G.; et al. Mussel-inspired synthesis of filter cotton-based AgNPs for oil/water separation, antibacterial and catalytic application. Mater. Today Commun. 2020, 25, 101467. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, F.; Chen, M.; Hu, H.; Lin, L.; Wu, J.; Zhang, M. Facile assembly of 2D α-zirconium phosphate supported silver nanoparticles: Superior and recyclable catalysis. New J. Chem. 2020, 44, 9793–9801. [Google Scholar] [CrossRef]
- Zheng, Y.; Qi, X.; Xiao, F.; Wang, F.; Wang, N. Spherical covalent organic frameworks supported black phosphorus@Au nanocatalysts for nitrophenol hydrogenation in a high efficiently flow-through process. Appl. Surf. Sci. 2023, 611, 155723. [Google Scholar] [CrossRef]
- Xu, Q.; Gao, T.; Zhang, S.; Zhang, M.; Li, X.; Liu, X. Synthesis of gold nanoparticle-loaded magnetic carbon microsphere based on reductive and binding properties of polydopamine for recyclable catalytic applications. New J. Chem. 2020, 44, 16227–16233. [Google Scholar] [CrossRef]
- Yang, X.; Lu, P.; Yu, L.; Pan, P.; Elzatahry, A.A.; Alghamdi, A.; Luo, W.; Cheng, X.; Deng, Y. An Efficient Emulsion-Induced Interface Assembly Approach for Rational Synthesis of Mesoporous Carbon Spheres with Versatile Architectures. Adv. Funct. Mater. 2020, 30, 2002488. [Google Scholar] [CrossRef]
- Zeng, T.; Zhang, X.; Niu, H.; Ma, Y.; Li, W.; Cai, Y. In situ growth of gold nanoparticles onto polydopamine-encapsulated magnetic microspheres for catalytic reduction of nitrobenzene. Appl. Catal. Environ. 2013, 134–135, 26–33. [Google Scholar] [CrossRef]
- Lai, G.; Zhang, H.; Yong, J.; Yu, A. In situ deposition of gold nanoparticles on polydopamine functionalized silica nanosphere for ultrasensitive nonenzymatic electrochemical immunoassay. Biosens. Bioelectron. 2013, 47, 178–183. [Google Scholar] [CrossRef]
- Zhou, J.; Duan, B.; Fang, Z.; Song, J.; Wang, C.; Messersmith, P.B.; Duan, H. Interfacial assembly of mussel-inspired Au@Ag@ polydopamine core–shell nanoparticles for recyclable nanocatalysts. Adv. Mater. 2014, 26, 701–705. [Google Scholar] [CrossRef]
- Ren, F.; Zhai, C.; Zhu, M.; Wang, C.; Wang, H.; Bin, D.; Guo, J.; Yang, P.; Du, Y. Facile synthesis of PtAu nanoparticles supported on polydopamine reduced and modified graphene oxide as a highly active catalyst for methanol oxidation. Electrochim. Acta 2015, 153, 175–183. [Google Scholar] [CrossRef]
- Rahoui, N.; Jiang, B.; Hegazy, M.; Taloub, N.; Wang, Y.; Yu, M.; Huang, Y.D. Gold modified polydopamine coated mesoporous silica nano-structures for synergetic chemo-photothermal effect. Colloids Surf. B 2018, 171, 176–185. [Google Scholar] [CrossRef]
- Acharya, A.; Kumar, A.; Lee, I.S. Yolk@Shell Nanoreactors Carrying a Cluster of Metal Nanocrystals Stabilized Inside the Hollow Carbon Shell. Bull. Korean Chem. Soc. 2021, 42, 915–918. [Google Scholar] [CrossRef]
- Zakia, M.; Yoo, S.I. Core–satellite assemblies of Au@polydopamine@Ag nanoparticles for photothermal-mediated catalytic reaction. Soft Matter 2020, 16, 10252–10259. [Google Scholar] [CrossRef]
- Xu, K.; Wu, J.; Fang, Q.; Bai, L.; Duan, J.; Li, J.; Xu, H.; Hui, A.; Hao, L.; Xuan, S. Magnetically separable h-Fe3O4@Au/polydopamine nanosphere with a hollow interior: A versatile candidate for nanocatalysis and metal ion adsorption. Chem. Eng. J. 2020, 398, 125571. [Google Scholar] [CrossRef]
- Hemmati, S.; Heravi, M.M.; Karmakar, B.; Veisi, H. In situ decoration of Au NPs over polydopamine encapsulated GO/Fe3O4 nanoparticles as a recyclable nanocatalyst for the reduction of nitroarenes. Sci. Rep. 2021, 11, 12362. [Google Scholar] [CrossRef]
- Liu, G.; Xiong, Q.; Xu, Y.; Fang, Q.; Leung, K.C.F.; Sang, M.; Xuan, S.; Hao, L. Sandwich-structured MXene@Au/polydopamine nanosheets with excellent photothermal-enhancing catalytic activity. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127860. [Google Scholar] [CrossRef]
- Lin, L.; Wen, Y.; Li, L.; Tan, Y.; Yang, P.; Liang, Y.; Xu, Y.; Hu, H.; Xu, Y. Mussel-Inspired Surface Modification of α-Zirconium Phosphate Nanosheets for Anchoring Efficient and Reusable Ultrasmall Au Nanocatalysts. Nanomaterials 2022, 12, 3339. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, W. Polydopamine nanospheres-grafted-PDMAEMA brushes/Au composites as a thermally adjustable catalyst for the reduction of 4-nitrophenol. Chem. Lett. 2022, 51, 811–814. [Google Scholar] [CrossRef]
- Ródenas, M.; Haskouri, J.E.; Ros-Lis, J.V.; Marcos, M.D.; Amorós, P.; Úbeda, M.Á.; Pérez-Pla, F. Highly active hydrogenation catalysts based on Pd nanoparticles dispersed along hierarchical porous silica covered with polydopamine as interfacial glue. Catalysts 2020, 10, 449. [Google Scholar] [CrossRef] [Green Version]
- Menumerov, E.; Hughes, R.A.; Neretina, S. Catalytic reduction of 4-nitrophenol: A quantitative assessment of the role of dissolved oxygen in determining the induction time. Nano Lett. 2016, 16, 7791–7797. [Google Scholar] [CrossRef]
- Strachan, J.; Barnett, C.; Masters, A.F.; Maschmeyer, T. 4-Nitrophenol Reduction: Probing the Putative Mechanism of the Model Reaction. ACS Catal. 2020, 10, 5516–5521. [Google Scholar] [CrossRef]
- Chen, X.; Yang, W.; Zhang, J.; Zhang, L.; Shen, H.; Shi, D. Alkalinity triggered the degradation of polydopamine nanoparticles. Polym. Bull. 2020, 78, 4439–4452. [Google Scholar] [CrossRef]














| Material | Formula | |
|---|---|---|
| PDA@Fe3O4 (thick film) | 27.4 | |
| C1 | 23.5 | ([Fe3O4]3.2 Ag)0.76(PDA H2O)0.24 |
| C2 | 20.6 | ([Fe3O4]6.3 Ag)0.79(PDA H2O)0.21 |
| C3 | 18.1 | ([Fe3O4]0.55 Ag)0.82(PDA H2O)0.18 |
| C4 | 24.1 | ([Fe3O4]3.3 Au)0.76(PDA H2O)0.24 |
| C5 | 18.7 | ([Fe3O4]8.8 Au)0.81(PDA H2O)0.19 |
| C6 | 20.9 | ([Fe3O4]12.5 Au)0.79(PDA H2O)0.21 |
| PDA@Fe3O4 (thin film) | 17.7 | |
| C7 | 13.4 | ([Fe3O4]6.02 Cu)0.87(PDA H2O)0.13 |
| C8 | 14.6 | ([Fe3O4]4.7 Ag)0.85(PDA H2O)0.15 |
| C9 | 17.6 | ([Fe3O4]1.6Ag Cu0.026)0.82(PDA H2O)0.18 |
| Catalyst | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 |
|---|---|---|---|---|---|---|---|---|---|
| Fe/M | 9.6 | 18.9 | 1.6 | 10.0 | 26.5 | 37.4 | 18.1 | 14.0 | (188.3 (Cu), 4.9 (Ag)) |
| Catalyst | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Surface/ | 37.4 | 40.9 | 26.9 | 38.5 | 40.2 | 39.1 | 27.8 | 51.2 | 46.9 |
| Pore Vol./ | 0.14 | 0.15 | 0.09 | 0.14 | 0.14 | 0.13 | 0.18 | 0.21 | 0.16 |
| Pore Size/nm | 26.7 | 25.4 | 29.8 | 21.1 | 20.2 | 25.0 | 35.9 | 31.2 | 35.3 |
| z/nm | pdi | |||||
|---|---|---|---|---|---|---|
| PDA@ (thick film) | 431 | 0.19 | 411 | 103.8 | – | – |
| 200 | 0.31 | 252 | 147.00 | – | – | |
| 333 | 0.34 | 442 | 129.00 | – | – | |
| 323 | 0.44 | 657 | 305.70 | 135.80 | 39.85 | |
| 249 | 0.33 | 299 | 154.20 | – | – | |
| 233 | 0.41 | 319 | 174.50 | 60.47 | 12.66 | |
| 232 | 0.40 | 389 | 141.60 | 98.56 | 28.39 | |
| PDA@ (thin film) | 210 | 0.20 | 242 | 90.02 | – | – |
| 470 | 0.41 | 432 | 158.2 | – | ||
| 198 | 0.19 | 240 | 97.08 | – | – |
| s | /h−1 | TOF /h−1M−1 | |||||
|---|---|---|---|---|---|---|---|
| 4-nitrophenol | |||||||
| C1 | 3 | 29 ± 2 | 765 ± 50 | 7400 ± 500 | 3.47 | 1.23 | 2.71 |
| C2 | 3 | 34 ± 8 | 680 ± 180 | 6600 ± 1700 | 4.02 | 0.98 | 5.32 |
| C3 | 3 | 28 ± 1 | 115 ± 1 | 1100 ± 200 | 3.57 | 1.24 | 2.68 |
| C4 | 3 | 22 ± 3 | 1400 ± 180 | 13,700 ± 1600 | 5.73 | 1.47 | 4.86 |
| C5 | 3 | 18 ± 2 | 820 ± 110 | 7900 ± 1100 | 5.07 | 1.83 | 1.78 |
| C6 | 2 | 144 ± 25 | 310 ± 50 | 3000 ± 500 | 7.80 | – | 2.68 |
| C7 | 3 | 26 ± 3 | 700 ± 70 | 6800 ± 700 | 5.51 | 0.98 | 43.6 |
| C8 | 3 | 22 ± 3 | 700 ± 100 | 6900 ± 900 | 6.61 | 1.63 | 6.64 |
| C9 | 2 | 55 ± 7 | 116 ± 15 | 1120 ± 140 | 4.82 | – | 14.0 |
| 3-nitrophenol | |||||||
| C1 | 2 | 23 ± 4 | 550 ± 110 | 5400 ± 1000 | 3.09 | – | 0.028 |
| C2 | 2 | 25 ± 4 | 930 ± 200 | 9000 ± 1800 | 3.26 | – | 1.09 |
| C3 | 2 | 18 ± 2 | 180 ± 25 | 1750.6 ± 220 | 4.11 | – | 0.63 |
| C4 | 3 | 9 ± 1 | 1700 ± 250 | 1600 ± 2300 | 12.4 | 1.96 | 1.94 |
| C5 | 2 | 132 ± 8 | 425 ± 15 | 4100 ± 150 | 1.05 | – | 0.90 |
| C6 | 2 | 300 ± 30 | 150 ± 15 | 1450 ± 130 | 0.23 | – | 0.00 |
| C7 | 2 | 15.2 ± 0.5 | 1225 ± 40 | 11,800 ± 400 | 4.73 | – | 0.32 |
| C9 | 2 | 80 ± 15 | 140 ± 5 | 1300 ± 50 | 1.89 | – | 2.33 |
| 2-nitrophenol | |||||||
| C1 | 2 | 27 ± 4 | 480 ± 70 | 4650 ± 750 | 2.86 | – | 0.002 |
| C2 | 2 | 31 ± 1 | 740 ± 20 | 7170 ± 140 | 2.92 | – | 2.11 |
| C3 | 2 | 28 ± 6 | 125 ± 25 | 1200 ± 250 | 3.02 | – | 1.28 |
| C4 | 2 | 40 ± 9 | 500 ± 125 | 4800 ± 1200 | 1.94 | – | 0.56 |
| C5 | 2 | 69 ± 15 | 480 ± 100 | 4600 ± 900 | 1.35 | – | 2.16 |
| C6 | 2 | 25 ± 8 | 2000 ± 600 | 19,000 ± 6000 | 2.61 | – | 0.00 |
| C7 | 2 | 15 ± 1 | 1270 ± 100 | 12,300 ± 900 | 5.05 | – | 0.58 |
| C9 | 2 | 47 ± 2 | 140 ± 5 | 1300 ± 50 | 1.89 | – | 2.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, H.K.; El Haskouri, J.; Marcos, M.D.; Ros-Lis, J.V.; Amorós, P.; Úbeda Picot, M.Á.; Pérez-Pla, F. Synthesis and Catalytic Activity for 2, 3, and 4-Nitrophenol Reduction of Green Catalysts Based on Cu, Ag and Au Nanoparticles Deposited on Polydopamine-Magnetite Porous Supports. Nanomaterials 2023, 13, 2162. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13152162
Brown HK, El Haskouri J, Marcos MD, Ros-Lis JV, Amorós P, Úbeda Picot MÁ, Pérez-Pla F. Synthesis and Catalytic Activity for 2, 3, and 4-Nitrophenol Reduction of Green Catalysts Based on Cu, Ag and Au Nanoparticles Deposited on Polydopamine-Magnetite Porous Supports. Nanomaterials. 2023; 13(15):2162. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13152162
Chicago/Turabian StyleBrown, Helen K., Jamal El Haskouri, María D. Marcos, José Vicente Ros-Lis, Pedro Amorós, M. Ángeles Úbeda Picot, and Francisco Pérez-Pla. 2023. "Synthesis and Catalytic Activity for 2, 3, and 4-Nitrophenol Reduction of Green Catalysts Based on Cu, Ag and Au Nanoparticles Deposited on Polydopamine-Magnetite Porous Supports" Nanomaterials 13, no. 15: 2162. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13152162