Ecotoxicological Effects of TiO2 P25 Nanoparticles Aqueous Suspensions on Zebrafish (Danio rerio) Eleutheroembryos
Abstract
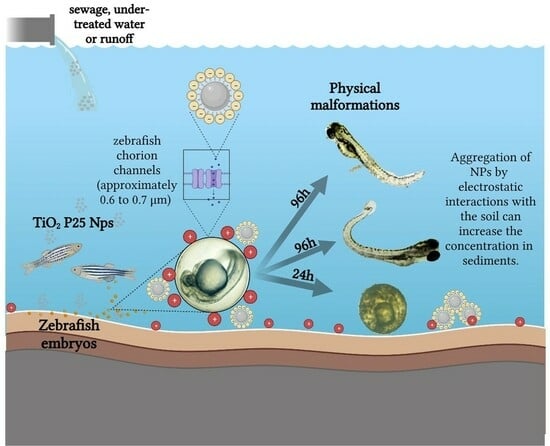
:1. Introduction
2. Materials and Methods
2.1. Fish Maintenance and Breeding: Zebrafish
2.2. Reproduction Process
2.3. Preparation of Aqueous Suspensions (TiO2 P25 Nanoparticles)
2.4. Characterization of TiO2 P25 Nanoparticles
2.5. Fish Embryo Acute Toxicity Test
2.6. Quantification of TiO2
2.7. Statistical Analysis
3. Results
3.1. Characterization of TiO2 P25 Nanoparticles
3.2. LC50 Values of TiO2 P25 Nanoparticles and Hatching Rate for Zebrafish Eleutheroembryos
3.3. Teratogenic Effects
3.4. Quantification of Ti4+ Concentration in Zebrafish Eleutheroembryos
4. Discussion
4.1. Characterization of TiO2 P25 Nanoparticles
4.2. LC50 Values of TiO2 P25 Nanoparticles and Hatching Rate for Zebrafish Eleutheroembryos
4.3. Teratogenic Effects
4.4. Quantification of Ti4+ Concentration in Zebrafish Eleutheroembryos
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef]
- Lomer, M.C.E.; Thompson, R.P.H.; Powell, J.J. Fine and ultrafine particles of the diet: Influence on the mucosal immune response and association with Crohn’s disease. Proc. Nutr. Soc. 2002, 61, 123–130. [Google Scholar] [CrossRef]
- Okuda-Shimazaki, J.; Takaku, S.; Kanehira, K.; Sonezaki, S.; Taniguchi, A. Effects of Titanium Dioxide Nanoparticle Aggregate Size on Gene Expression. Int. J. Mol. Sci. 2010, 11, 2383–2392. [Google Scholar] [CrossRef]
- Ozkan, Y.; Altinok, I.; Ilhan, H.; Sokmen, M. Determination of TiO2 and AgTiO2 Nanoparticles in Artemia salina: Toxicity, Morphological Changes, Uptake and Depuration. Bull. Environ. Contam. Toxicol. 2016, 96, 36–42. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Shah, S.N.A.; Shah, Z.; Hussain, M.; Khan, M. Hazardous Effects of Titanium Dioxide Nanoparticles in Ecosystem. Bioinorg. Chem. Appl. 2017, 4101735. [Google Scholar] [CrossRef] [PubMed]
- Kiser, M.A.; Westerhoff, P.; Benn, T.; Wang, Y.; Pérez-Rivera, J.; Hristovski, K. Titanium Nanomaterial Removal and Release from Wastewater Treatment Plants. Environ. Sci. Technol. 2009, 43, 6757–6763. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, L.; Chen, F.; Zou, H.; Wang, Z. A key moment for TiO2: Prenatal exposure to TiO2 nanoparticles may inhibit the development of offspring. Ecotoxicol. Environ. Saf. 2020, 202, 110911. [Google Scholar] [CrossRef] [PubMed]
- Virkutyte, J.; Al-Abed, S.R.; Dionysiou, D.D. Depletion of the protective aluminum hydroxide coating in TiO2-based sunscreens by swimming pool water ingredients. Chem. Eng. J. 2012, 191, 95–103. [Google Scholar] [CrossRef]
- Peters, R.J.B.; van Bemmel, G.; Herrera-Rivera, Z.; Helsper, H.P.F.G.; Marvin, H.J.P.; Weigel, S.; Tromp, P.C.; Oomen, A.G.; Rietveld, A.G.; Bouwmeester, H. Characterisation of titanium dioxide nanoparticles in food products: Analytical methods to define nanoparticles. J. Agric. Food Chem. 2014, 62, 6285–6293. [Google Scholar] [CrossRef] [PubMed]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S.; et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017, 7, srep40373. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Fang, T.; Yu, L.; Sima, X.; Zhu, W. Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: Acute toxicity, oxidative stress and oxidative damage. Sci. Total. Environ. 2011, 409, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-J.; Su, C.-C.; Chen, C.-W.; Dong, C.-D.; Liu, W.-S.; Huang, C. Adsorption characteristics of nano-TiO2 onto zebrafish embryos and its impacts on egg hatching. Chemosphere 2016, 154, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Aleström, P.; D’angelo, L.; Midtlyng, P.J.; Schorderet, D.F.; Schulte-Merker, S.; Sohm, F.; Warner, S. Zebrafish: Housing and husbandry recommendations. Lab. Anim. 2020, 54, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Adhish, M.; Manjubala, I. Effectiveness of zebrafish models in understanding human diseases—A review of models. Heliyon 2023, 9, e14557. [Google Scholar] [CrossRef]
- Hamlin, H.J.; Guillette, L.J. Birth Defects in Wildlife: The Role of Environmental Contaminants as Inducers of Reproductive and Developmental Dysfunction. Syst. Biol. Reprod. Med. 2010, 56, 113–121. [Google Scholar] [CrossRef]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a Model Vertebrate for Investigating Chemical Toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef]
- Díaz, P.; Powell, T.L.; Jansson, T. The Role of Placental Nutrient Sensing in Maternal-Fetal Resource Allocation1. Biol. Reprod. 2014, 91, 82. [Google Scholar] [CrossRef]
- Wang, Y.-J.; He, Z.-Z.; Fang, Y.-W.; Xu, Y.; Chen, Y.-N.; Wang, G.-Q.; Yang, Y.-Q.; Yang, Z.; Li, Y.-H. Effect of titanium dioxide nanoparticles on zebrafish embryos and developing retina. Int. J. Ophthalmol. 2014, 7, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Clemente, Z.; Castro, V.; Moura, M.; Jonsson, C.; Fraceto, L. Toxicity assessment of TiO2 nanoparticles in zebrafish embryos under different exposure conditions. Aquat. Toxicol. 2014, 147, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.; Navas, J.M.; Soares, A.M.; Barata, C. Oxidative stress effects of titanium dioxide nanoparticle aggregates in zebrafish embryos. Sci. Total. Environ. 2014, 470–471, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Khoshnood, R.; Jaafarzadeh, N.; Jamili, S.; Farshchi, P.; Taghavi, L. Acute toxicity of TiO2, CuO and ZnO nanoparticles in brine shrimp, Artemia franciscana. Iran. J. Fish. Sci. 2017, 16, 1287–1296. [Google Scholar]
- Dağlioğlu, Y.; Altinok, İ.; İlhan, H.; Sökmen, M. Determination of the acute toxic effect of ZnO-TiO2 nanoparticles in brine shrimp (Artemia salina). Acta Biol. Turc. 2018, 29, 6–13. [Google Scholar]
- Ates, M.; Daniels, J.; Arslan, Z.; Farah, I.O. Effects of aqueous suspensions of titanium dioxide nanoparticles on Artemia salina: Assessment of nanoparticle aggregation, accumulation, and toxicity. Environ. Monit. Assess. 2013, 185, 3339–3348. [Google Scholar] [CrossRef]
- Rohit, R.; Murthy, C.L.N.; Idris, M.M.; Singh, S. Toxicity of TiO2, SiO2, ZnO, CuO, Au and Ag engineered nanoparticles on hatching and early nauplii of Artemia sp. bioRxiv 2018. [Google Scholar] [CrossRef]
- Samaee, S.-M.; Rabbani, S.; Jovanović, B.; Mohajeri-Tehrani, M.R.; Haghpanah, V. Efficacy of the hatching event in assessing the embryo toxicity of the nano-sized TiO2 particles in zebrafish: A comparison between two different classes of hatching-derived variables. Ecotoxicol. Environ. Saf. 2015, 116, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.; Lewis, C.; Dolciotti, I.; Johnston, B.D.; Moger, J.; Regoli, F. Sublethal toxicity of nano-titanium dioxide and carbon nanotubes in a sediment dwelling marine polychaete. Environ. Pollut. 2010, 158, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2023; pp. 1–22. [Google Scholar] [CrossRef]
- Hoo, J.Y.; Kumari, Y.; Shaikh, M.F.; Hue, S.M.; Goh, B.H. Zebrafish: A Versatile Animal Model for Fertility Research. BioMed Res. Int. 2016, 2016, 9732780. [Google Scholar] [CrossRef]
- Behnajady, M.; Yavari, S.; Modirshahla, N. Ispitivanje adsorpcionog kapaciteta TiO2-P25 nanočestica za uklanjanje mono-azo boje iz vodenog rastvora: Sveobuhvatna analiza izotermi. Chem. Ind. Chem. Eng. Q. 2014, 20, 97–107. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. Available online: https://0-academic-oup-com.brum.beds.ac.uk/aje/article-abstract/27/3/493/99616 (accessed on 8 January 2023). [CrossRef]
- Morgan, R.; Kundomal, Y.; Hupp, E. SAS probit analysis for cadmium mortality. Environ. Res. 1982, 29, 233–237. [Google Scholar] [CrossRef]
- Clemente, Z.; Castro, V.L.; Jonsson, C.M.; Fraceto, L.F. Minimal levels of ultraviolet light enhance the toxicity of TiO2 nanoparticles to two representative organisms of aquatic systems. J. Nanopart. Res. 2014, 16, 8. [Google Scholar] [CrossRef]
- Li, L. Fate and Biological Effects of Titanium Dioxide Nanoparticles in the Aquatic Environment. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2017. Available online: http://ethesis.helsinki.fi (accessed on 6 January 2024).
- French, R.A.; Jacobson, A.R.; Kim, B.; Isley, S.L.; Penn, R.L.; Baveye, P.C. Influence of Ionic Strength, pH, and Cation Valence on Aggregation Kinetics of Titanium Dioxide Nanoparticles. Environ. Sci. Technol. 2009, 43, 1354–1359. [Google Scholar] [CrossRef]
- Pockberger, M.; Kellnreitner, F.; Ahnelt, H.; Asmus, R.; Asmus, H. An abundant small sized fish as keystone species? The effect of Pomatoschistus microps on food webs and its trophic role in two intertidal benthic communities: A modeling approach. J. Sea Res. 2014, 86, 86–96. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, L.; Duan, Z.; Qi, R.; Li, Y.; Lang, Y. Comparative toxicity of several metal oxide nanoparticle aqueous suspensions to Zebrafish (Danio rerio) early developmental stage. J. Environ. Sci. Health Part A 2008, 43, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Lin, B.; Hu, C.; Zhang, H.; Lin, Z.; Xi, Z. The combined toxicological effects of titanium dioxide nanoparticles and bisphenol A on zebrafish embryos. Nanoscale Res. Lett. 2014, 9, 406. [Google Scholar] [CrossRef]
- Johnson, R.C.; Stewart, A.R.; Limburg, K.E.; Huang, R.; Cocherell, D.; Feyrer, F. Lifetime Chronicles of Selenium Exposure Linked to Deformities in an Imperiled Migratory Fish. Environ. Sci. Technol. 2020, 54, 2892–2901. [Google Scholar] [CrossRef]
- Gilbert Scott, F. PART 1. Principles of development in biology. In Developmental Biology; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Wang, Z.; Wang, Z. Nanoparticles induced embryo–fetal toxicity. Toxicol. Ind. Health 2020, 36, 181–213. [Google Scholar] [CrossRef]
- Li, C.; Tang, M. The toxicological effects of nano titanium dioxide on target organs and mechanisms of toxicity. J. Appl. Toxicol. 2023, 44, 152–164. [Google Scholar] [CrossRef]
- Geiser, M.; Casaulta, M.; Kupferschmid, B.; Schulz, H.; Semmler-Behnke, M.; Kreyling, W. The Role of Macrophages in the Clearance of Inhaled Ultrafine Titanium Dioxide Particles. Am. J. Respir. Cell Mol. Biol. 2008, 38, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Mugoni, V.; Camporeale, A.; Santoro, M.M. Analysis of Oxidative Stress in Zebrafish Embryos. J. Vis. Exp. 2014, 89, e51328. [Google Scholar] [CrossRef]
- Parlak, V. Evaluation of apoptosis, oxidative stress responses, AChE activity and body malformations in zebrafish (Danio rerio) embryos exposed to deltamethrin. Chemosphere 2018, 207, 397–403. [Google Scholar] [CrossRef]
- Ze, Y.; Zheng, L.; Zhao, X.; Gui, S.; Sang, X.; Su, J.; Guan, N.; Zhu, L.; Sheng, L.; Hu, R.; et al. Molecular mechanism of titanium dioxide nanoparticles-induced oxidative injury in the brain of mice. Chemosphere 2013, 92, 1183–1189. [Google Scholar] [CrossRef]
- Pietroiusti, A.; Massimiani, M.; Fenoglio, I.; Colonna, M.; Valentini, F.; Palleschi, G.; Camaioni, A.; Magrini, A.; Siracusa, G.; Bergamaschi, A.; et al. Low Doses of Pristine and Oxidized Single-Wall Carbon Nanotubes Affect Mammalian Embryonic Development. ACS Nano 2011, 5, 4624–4633. [Google Scholar] [CrossRef] [PubMed]
- Raldúa, D.; André, M.; Babin, P.J. Clofibrate and gemfibrozil induce an embryonic malabsorption syndrome in zebrafish. Toxicol. Appl. Pharmacol. 2008, 228, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Gao, H.-W.; Wu, L.-L. Transmembrane Transport of Microcystin to Danio rerio Zygotes: Insights into the Developmental Toxicity of Environmental Contaminants. Toxicol. Sci. 2011, 122, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Kodde, I.F.; van der Stok, J.; Smolenski, R.T.; de Jong, J.W. Metabolic and genetic regulation of cardiac energy substrate preference. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 146, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Le Guellec, D.; Morvan-Dubois, G.; Sire, J.-Y. Skin development in bony fish with particular emphasis on collagen deposition in the dermis of the zebrafish (Danio rerio). Int. J. Dev. Biol. 2004, 48, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Voices of the Bay. Fishery Science-Biology & Ecology. 2011. Available online: https://nmssanctuaries.blob.core.windows.net/sanctuaries-prod/media/archive/education/voicesofthebay/pdfs/howfisheat.pdf (accessed on 6 January 2024).







| Organism | Title/Authors | Material and Concentrations | Conclusions |
|---|---|---|---|
| Zebrafish embryos | Effect of titanium dioxide nanoparticles on zebrafish embryos and developing retina [19] | Commercial TiO2 NPs (P-25 type, 21 nm average size) 20, 10, 5, 1, 0.75, 0.5, and 0.2 mg/L |
|
| Zebrafish Embryos | Toxicity assessment of TiO2 nanoparticles in zebrafish embryos under different exposure conditions [20] | Commercial TiO2 NPs (P-25 type, 25 nm average size) 1, 10, and 100 mg/L |
|
| Zebrafish embryos | Oxidative stress effects of titanium dioxide nanoparticle aggregates in zebrafish embryos [21] | TiO2 (20 nm), P25, Micro (200 nm) 0.01, 0.1, and 1 mg/mL |
|
| Brine shrimp (Artemia franciscana) | Acute toxicity of TiO2, CuO, and ZnO nanoparticles in brine shrimp, Artemia franciscana [22] | TiO2 NPs 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 mg/L |
|
| Brine shrimp (Artemia salina) | Determination of the acute toxic effect of ZnO-TiO2 nanoparticles in brine shrimp (Artemia salina) [23] | TiO2 (99.0% pure) was obtained in pure anatase. |
|
| Brine shrimp (Artemia salina) | Effects of aqueous suspensions of titanium dioxide nanoparticles on Artemia salina: Assessment of nanoparticle aggregation, accumulation, and toxicity [24] | TiO2 NPs, (99.5% rutile polymorph) 10, 50, and 100 mg/L |
|
| Early nauplii of (Artemia sp.) | Toxicity of TiO2, SiO2, ZnO, CuO, Au, and Ag-engineered nanoparticles on hatching and early nauplii of Artemia sp. [25] | Nano-TiO2 (synthesized) 1, 10, and 100 mg/L |
|
| Zebrafish embryos | Adsorption characteristics of nano-TiO2 onto zebrafish embryos and its impacts on egg hatching [13] | Nano-TiO2 P-25 0, 10, 20, 60, and 120 mg/L |
|
| Zebrafish embryos | Efficacy of the hatching event in assessing the embryo toxicity of the nano-sized TiO2 particles in zebrafish: A comparison between two different classes of hatching-derived variables [26] | P-25 nTiO2 21 nm 0, 0.01, 10, and 1000 mg/mL |
|
| LC20 (mg/L) | LC30 (mg/L) | LC50 (mg/L) | LC80 (mg/L) | |
|---|---|---|---|---|
| Graph estimation | 188.34 ± 19.38 | 224.34 ± 17.46 | 269.60 ± 14.88 | n.a. |
| Exponential equation | 188.25 ± 20.51 | 227.46 ± 25.55 | 288.90 ± 36.44 | 360.23 ± 52.56 |
| Probit | 171.62 ± 20.48 | 230.50 ± 31.38 | 345.47 ± 57.23 | 665.84 ± 148.30 |
| Origin | 184.70 ± 21.49 | n.a. | 293.49 ± 0 | 470.92 ± 58.50 |
| Average | 183.23 ± 7.92 | 227.43 ± 3.08 | 301.32 ± 39.43 | 499.00 ± 154.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Román, M.I.; Casiano-Muñiz, I.M.; Román-Velázquez, F.R. Ecotoxicological Effects of TiO2 P25 Nanoparticles Aqueous Suspensions on Zebrafish (Danio rerio) Eleutheroembryos. Nanomaterials 2024, 14, 373. https://0-doi-org.brum.beds.ac.uk/10.3390/nano14040373
Ortiz-Román MI, Casiano-Muñiz IM, Román-Velázquez FR. Ecotoxicological Effects of TiO2 P25 Nanoparticles Aqueous Suspensions on Zebrafish (Danio rerio) Eleutheroembryos. Nanomaterials. 2024; 14(4):373. https://0-doi-org.brum.beds.ac.uk/10.3390/nano14040373
Chicago/Turabian StyleOrtiz-Román, Melissa I., Ileska M. Casiano-Muñiz, and Felix R. Román-Velázquez. 2024. "Ecotoxicological Effects of TiO2 P25 Nanoparticles Aqueous Suspensions on Zebrafish (Danio rerio) Eleutheroembryos" Nanomaterials 14, no. 4: 373. https://0-doi-org.brum.beds.ac.uk/10.3390/nano14040373