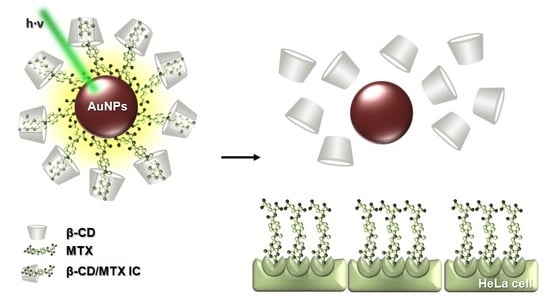
Photothermally Controlled Methotrexate Release System Using β-Cyclodextrin and Gold Nanoparticles
Abstract
:1. Introduction
2. Experimental
2.1. Purification of MTX
2.2. Preparation and Characterization of the β-CD/MTX Inclusion Compound
2.3. Synthesis and Characterization of Colloidal AuNPs and Their Conjugation with the β-CD/MTX
2.4. Evaluation of the Effect of MTX and Its Derivatives on Cell Viability
2.4.1. Cell Culture and Cells
2.4.2. Cell Viability Determination by MTS Assay
2.4.3. Effect of the Irradiated AuNPs + β-CD/MTX on Cell Viability
2.4.4. Data Analysis
3. Results and Discussion
3.1. Characterization of β-CD/MTX
3.2. Obtaining and Characterizing the Ternary System AuNPs+β-CD/MTX
3.3. Evaluation of the Effect of MTX and Its Derivatives on Cell Viability
3.4. Evaluation of Cell Viability by MTS Assays of AuNPs + β-CD/MTX Samples with and without Irradiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Llevot, A.; Astruc, D. Applications of vectorized gold nanoparticles to the diagnosis and therapy of cancer. Chem. Soc. Rev. 2012, 41, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S.; Ahmad, M.Z.; Ahmad, F.J.; Storm, G.; Kok, R.J. Gold nanoparticles in theranostic oncology: Current state-of-the-art. Expert Opin. Drug Deliv. 2012, 9, 1225–1243. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. WIREs Nanomed. Nanobiotechnol. 2017, 9, 1449–1465. [Google Scholar] [CrossRef] [PubMed]
- Haume, K.; Rosa, S.; Grellet, S.; Śmiałek, M.A.; Butterworth, K.T.; Solov’yov, A.V.; Prise, K.M.; Golding, J.; Mason, N.J. Gold nanoparticles for cancer radiotherapy: A review. Cancer Nanotechnol. 2016, 7, 8–28. [Google Scholar] [CrossRef] [PubMed]
- Abadeer, N.S.; Murphy, C.J. Recent progress in cancer thermal therapy using gold nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Inostroza-Riquelme, M.; Vivanco, A.; Lara, P.; Guerrero, S.; Salas-Huenuleo, E.; Chamorro, A.; Leyton, L.; Bolaños, K.; Araya, E.; Quest, A.F.G.; et al. Encapsulation of Gold Nanostructures and Oil-in-Water Nanocarriers in Microgels with Biomedical Potential. Molecules 2018, 23, 1208. [Google Scholar] [CrossRef] [PubMed]
- Vetterlein, C.; Vásquez, R.; Bolaños, K.; Acosta, G.A.; Guzman, F.; Albericio, F.; Celis, F.; Campos, M.; Kogan, M.J.; Araya, E. Exploring the influence of Diels–Alder linker length on photothermal molecule release from gold nanorods. Colloids Surf. B Biointerfaces 2018, 166, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Sierpe, R.; Noyong, M.; Simond, U.; Aguayo, D.; Huerta, J.; Kogan, M.; Yutronic, N. Construction of 6 thioguanine and 6-mercaptopurine carriers based on βcyclodextrins and gold nanoparticles. Carbohydr. Polym. 2017, 177, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Asela, I.; Noyong, M.; Simon, U.; Andrades-Lagos, J.; Campanini-Salinas, J.; Vásquez-Velásquez, D.; Kogan, M.; Yutronic, N.; Sierpe, R. Gold nanoparticles stabilized with βcyclodextrin-2-amino-4-(4-chlorophenyl)thiazole complex: A novel system for drug transport. PLoS ONE 2017, 12, e0185652. [Google Scholar] [CrossRef] [PubMed]
- Hagner, N.; Joerger, M. Cancer chemotherapy: Targeting folic acid synthesis. Cancer Manag. Res. 2010, 19, 293–301. [Google Scholar]
- Herfarth, H.H.; Kappelman, M.D.; Long, M.D.; Isaacs, K.L. Use of Methotrexate in the Treatment of Inflammatory Bowel Diseases. Inflammat. Bowel Dis. 2016, 22, 224–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, E.S.L.; Cronstein, B.N. Methotrexate-how does it really work? Nat. Rev. Rheumatol. 2010, 6, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Feng, Y.; Yu, Y.; Sun, W.; Bai, J.; Chen, F.; Fu, S. The molecular mechanism of resistance to methotrexate in mouse methotrexate-resistant cells by cancer drug resistance and metabolism SuperArray. Basic Clin. Pharmacol. Toxicol. 2006, 99, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Aithal, U.V.; Udupa, K.S. Physicochemical and biological studies of inclusion complex of methotrexate with β-cyclodextrin. Pharm. Sci. 1997, 3, 573–577. [Google Scholar]
- Pattarino, M.T.F.; Giovannelli, L.; Giovenzana, G.B.; Rinaldi, M. Inclusion of methotrexate in alkyl-cyclodextrins: Effects of host substitutents on the stability of complexes. J. Drug Deliv. Sci. Technol. 2005, 15, 465–468. [Google Scholar] [CrossRef]
- Kritskiy, I.; Kumeev, R.; Volkova, T.; Shipilov, D.; Kutyasheva, N.; Grachev, M.; Terekhova, I. Selective binding of methotrexate to monomeric, dimeric and polymeric cyclodextrins. New J. Chem. 2018, 17, 14559–14567. [Google Scholar] [CrossRef]
- Chen, Y.; Tsai, C.; Huang, P.; Chang, M.; Cheng, P.; Chou, C.; Chen, D.; Wang, C.; Shiau, A.; Wu, C. Methotrexate Conjugated to Gold Nanoparticles Inhibits Tumor Growth in a Syngeneic Lung Tumor Model. Mol. Pharm. 2007, 4, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Bessar, H.; Venditti, L.; Fratoddi, I.; Benassi, L.; Vaschieri, C.; Azzoni, P.; Pellacani, G.; Magnoni, C.; Botti, E.; Casagrande, B.; et al. Functionalized gold nanoparticles for topical delivery of methotrexate for the possible treatment of psoriasis. Colloids Surf. B Biointerfaces 2016, 141, 141–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, N.T.T.; Wang, T.H.; Lin, C.Y.; Tai, Y. Synthesis of methotrexate-conjugated gold nanoparticles with enhanced cancer therapeutic effect. Biochem. Eng. J. 2013, 78, 175–180. [Google Scholar] [CrossRef]
- Wang, W.Y.; Zhao, X.F.; Ju, X.H.; Wang, Y.; Wang, L.; Li, S.P.; Li, X.D. Novel morphology change of Au-Methotrexate conjugates: From nanochains to discrete nanoparticles. Int. J. Pharm. 2016, 515, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Aykaç, K.; Martos-Maldonado, M.C.; Casas-Solvas, J.M.; Quesada-Soriano, I.; García-Maroto, F.; García-Fuentes, L.; Vargas-Berenguel, A. β-Cyclodextrin-bearing gold glyconanoparticles for the development of site specific drug delivery systems. Langmuir 2014, 30, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Muñoz, C.; Diaz-Marcos, J.; Samitier, J.; Yutronic, N.; Kogan, M.J.; Jara, P. In situ visualization of the local photothermal effect produced on α-cyclodextrin inclusion compound associated with gold nanoparticles. Nanoscale Res. Lett. 2016, 11, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Moris, S.; Díaz, M.; Yutronic, N.; Lang, E.; Chornik, B.; Kogan, M.J.; Jara, P. Evidence of the disassembly of α-cyclodextrin-octylamine inclusion compounds conjugated to gold nanoparticles via thermal and photothermal effects. Molecules 2016, 21, 1444. [Google Scholar] [CrossRef] [PubMed]
- Sierpe, R.; Lang, E.; Jara, P.; Guerrero, A.R.; Chornik, B.; Kogan, M.J.; Yutronic, N. Gold Nanoparticles Interacting with β-Cyclodextrin–phenylethylamine inclusion complex: A ternary system for photothermal drug release. ACS Appl. Mater. Interfaces 2015, 7, 15177–15188. [Google Scholar] [CrossRef] [PubMed]
- Jayalakshmi, K.; Ghoshal, U.C.; Kumar, S.; Misra, A.; Roy, R.; Khetrapal, C.L. Assessment of small intestinal permeability using 1H-NMR spectroscopy. J. Gastrointest. Liver Dis. 2009, 18, 27–32. [Google Scholar]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. The size and shape factor in colloidal systems. J. Phys. Chem. 1953, 57, 670–673. [Google Scholar] [CrossRef]
- Ghosh, S.K. Spectroscopic evaluation of 4-(dimethylamino)pyridine versus citrate as stabilizing ligand for gold nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2010, 371, 98–103. [Google Scholar] [CrossRef]
- Han, S.W.; Joo, S.W.; Ha, T.H.; Kim, Y.; Kim, K. Adsorption characteristics of anthraquinone-2-carboxylic acid on gold. J. Phys. Chem. B 2000, 104, 11987–11995. [Google Scholar] [CrossRef]
- Al-Johani, H.; Abou-Hamad, E.; Jedidi, A.; Widdifield, C.M.; Viger-Gravel, J.; Sangaru, S.S.; Gajan, D.; Anjum, D.H.; Ould-Chikh, S.; Hedhili, M.N.; et al. The structure and binding mode of citrate in the stabilization of gold nanoparticles. Nat. Chem. 2017, 9, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues de Oliveira, A.; Ferreira Molina, E.; de Castro Mesquita, P.; Cardozo Fonseca, J.L.; Rossanezi, G.; Fernandes-Pedrosa, M.F.; Gomes de Oliveira, A.; da Silva-Júnior, A.A. Structural and thermal properties of spray-dried methotrexate-loaded biodegradable microparticles. J. Therm. Anal. Calorim. 2013, 112, 555–565. [Google Scholar] [CrossRef]
- Barbosa, J.A.A.; Zoppi, A.; Quevedo, M.A.; de Melo, P.N.; de Medeiros, A.S.A.; Streck, L.; de Oliveira, A.R.; Fernandes-Pedrosa, M.F.; Longhi, M.R.; da Silva-Júnior, A.A. Triethanolamine Stabilization of Methotrexate β-Cyclodextrin Interactions in Ternary Complexes. Int. J. Mol. Sci. 2014, 15, 17077–17099. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Xiao, Z.; Zhou, R.; Zhu, G.; Niu, Y. Kinetics and release characteristics of menthyl acetate from its β-cyclodextrin inclusion complex by thermogravimetric analysis. J. Incl. Phenomena Macrocycl. Chem. 2016, 84, 219–224. [Google Scholar] [CrossRef]
- Rosowsky, A.; Galivan, J.; Beardsley, G.P.; Bader, H.; O’Connor, B.M.; Russello, O.; Moroson, B.A.; DeYarman, M.T.; Kerwar, S.S.; Freisheim, J.H. Biochemical and biological studies on 2-desamino-2-methylaminopterin, an antifolate the polyglutamates of which are more potent than the monoglutamate against three key enzymes of folate metabolism. Cancer Res. 1992, 52, 2148–2155. [Google Scholar] [PubMed]
- Goodsell, D.S. The molecular perspective: Methotrexate. Oncologist 1999, 4, 340–341. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Peuhu, E.; Bate-Eya, L.T.; Eriksson, J.E.; Sahlgren, C.; Lindén, M. Cancer-Cell-Specific Induction of Apoptosis Using Mesoporous Silica Nanoparticles as Drug-Delivery Vectors. Small 2010, 6, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Rashid, S.; Nafees, S.; Hasan, S.K.; Sultana, S. Beneficial effects of Chrysin against Methotrexate induced hepatotoxicity via attenuation of oxidative stress and apoptosis. Mol. Cell. Biochem. 2014, 385, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Sramek, M.; Neradil, J.; Veselska, R. Much more than you expected: The non-DHFR-mediated effects of methotrexate. Biochimica et Biophysica Acta 2017, 1861, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hussein, O.E.; Hozayen, W.G.; Abd El-Twab, S.M. Methotrexate hepatotoxicity is associated with oxidative stress, and down-regulation of PPARγ and Nrf2: Protective effect of 18β-Glycyrrhetinic acid. Chem.-Biol. Interact. 2017, 270, 59–72. [Google Scholar] [CrossRef] [PubMed]










© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, N.; Riveros, A.; Yutronic, N.; Lang, E.; Chornik, B.; Guerrero, S.; Samitier, J.; Jara, P.; Kogan, M.J. Photothermally Controlled Methotrexate Release System Using β-Cyclodextrin and Gold Nanoparticles. Nanomaterials 2018, 8, 985. https://0-doi-org.brum.beds.ac.uk/10.3390/nano8120985
Silva N, Riveros A, Yutronic N, Lang E, Chornik B, Guerrero S, Samitier J, Jara P, Kogan MJ. Photothermally Controlled Methotrexate Release System Using β-Cyclodextrin and Gold Nanoparticles. Nanomaterials. 2018; 8(12):985. https://0-doi-org.brum.beds.ac.uk/10.3390/nano8120985
Chicago/Turabian StyleSilva, Nataly, Ana Riveros, Nicolás Yutronic, Erika Lang, Boris Chornik, Simón Guerrero, Josep Samitier, Paul Jara, and Marcelo J. Kogan. 2018. "Photothermally Controlled Methotrexate Release System Using β-Cyclodextrin and Gold Nanoparticles" Nanomaterials 8, no. 12: 985. https://0-doi-org.brum.beds.ac.uk/10.3390/nano8120985