A Comparison of Hydrogen Storage in Pt, Pd and Pt/Pd Alloys Loaded Disordered Mesoporous Hollow Carbon Spheres
Abstract
:1. Introduction
2. Material and Methods
2.1. Synthesis of Disordered Mesoporous Hollow Carbon Spheres (HCS)
2.2. Synthesis of HCS Modified with Metal Nanoparticles
2.3. Characterization
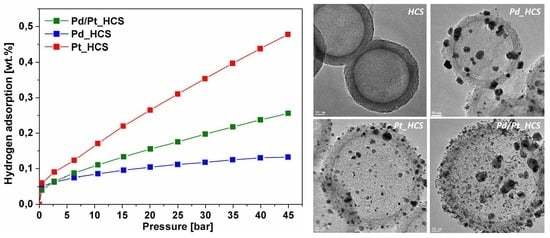
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadie, N.P.; Purewal, J.J.; Ahn, C.C.; Fultz, B. Measurements of hydrogen spillover in platinum doped superactivated carbon. Langmuir 2010, 26, 15481–15485. [Google Scholar] [CrossRef] [PubMed]
- Jordá-Beneyto, M.; Suárez-García, F.; Lozano-Castelló, D.; Cazorla-Amorós, D.; Linares-Solano, A. Hydrogen storage on chemically activated carbons and carbon nanomaterials at high pressures. Carbon 2007, 45, 293–303. [Google Scholar] [CrossRef]
- Juan-Juan, J.; Marco-Lozar, J.P.; Suárez-García, F.; Cazorla-Amorós, D.; Linares-Solano, A. A comparison of hydrogen storage in activated carbons and a metal-organic framework (MOF-5). Carbon 2010, 48, 2906–2909. [Google Scholar] [CrossRef]
- Sethia, G.; Sayari, A. Activated carbon with optimum pore size distribution for hydrogen storage. Carbon 2016, 99, 289–294. [Google Scholar] [CrossRef]
- Liu, C.; Fan, Y.Y.; Liu, M.; Cong, H.T.; Cheng, H.M.; Dresselhaus, M.S. Hydrogen storage in single-walled carbon nanotubes at room temperature. Science 1999, 286, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, M.; Takenobu, T.; Kataura, H.; Ata, M. Hydrogen adsorption and desorption in carbon nanotube systems and its mechanisms. Appl. Phys. A 2004, 78, 947–953. [Google Scholar] [CrossRef]
- Ye, Y.; Ahn, C.C.; Witham, C.; Fultz, B.; Liu, J.; Rinzler, A.G.; Colbert, D.; Smith, K.A.; Smalley, R.E. Hydrogen adsorption and cohesive energy of single-walled carbon nanotubes. Appl. Phys. Lett. 1999, 74, 2307–2309. [Google Scholar] [CrossRef] [Green Version]
- Mu, S.C.; Tang, H.L.; Qian, S.H.; Pan, M.; Yuan, R.Z. Hydrogen storage in carbon nanotubes modified by microwave plasma etching and Pd decoration. Carbon 2006, 44, 762–767. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Wu, C.-Z.; Xu, S.-T.; Cheng, H.-M. Hydrogen storage in carbon nanotubes revisited. Carbon 2010, 48, 452–455. [Google Scholar] [CrossRef]
- Marella, M.; Tomaselli, M. Synthesis of carbon nanofibers and measurements of hydrogen storage. Carbon 2006, 44, 1404–1413. [Google Scholar] [CrossRef]
- Liu, Y.; Brown, C.M.; Neumann, D.A.; Geohegan, D.B.; Puretzky, A.A.; Rouleau, C.M.; Hu, H.; Styers-Barnett, D.; Krasnov, P.O.; Yakobson, B.I. Metal-assisted hydrogen storage on Pt-decorated single-walled carbon nanohorns. Carbon 2012, 50, 4953–4964. [Google Scholar] [CrossRef]
- Gaboardi, M.; Amadé, S.N.; Aramini, M.; Milanese, C.; Magnani, G.; Sanna, S.; Riccò, M.; Pontiroli, D. Extending the hydrogen storage limit in fullerene. Carbon 2017, 120, 77–82. [Google Scholar] [CrossRef]
- Lachawiec, A.J.; Qi, G.; Yang, R.T. Hydrogen storage in nanostructured carbons by spillover: Bridge-building enhancement. Langmuir 2005, 21, 11418–11424. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, R.T. Hydrogen storage in metal-organic frameworks by bridged hydrogen spillover. J. Am. Chem. Soc. 2006, 128, 8136–8137. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, R.T. Hydrogen storage in low silica type X zeolites. J. Phys. Chem. B 2006, 110, 17175–17181. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Deng, S. Hydrogen adsorption on ordered mesoporous carbons doped with Pd, Pt, Ni, and Ru. Langmuir 2009, 25, 12550–12560. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, L.; Yang, J.; Yang, R.T. Investigation on hydrogenation of metal-organic frameworks HKUST-1, MIL-53, and ZIF-8 by hydrogen spillover. J. Phys. Chem. C 2013, 117, 7565–7576. [Google Scholar] [CrossRef]
- Rossetti, I.; Ramis, G.; Gallo, A.; Di Michele, A. Hydrogen storage over metal-doped activated carbon. Int. J. Hydrogen Energy 2015, 40, 7609–7616. [Google Scholar] [CrossRef]
- Jin, J.; Ouyang, J.; Yang, H. Pd Nanoparticles and MOFs Synergistically hybridized halloysite nanotubes for hydrogen storage. Nanoscale Res. Lett. 2017, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Gong, Q.; Zhao, Y.; Li, J.; Lueking, A.D. Stability and hydrogen adsorption of metal-organic frameworks prepared via different catalyst doping methods. J. Catal. 2014, 318, 128–142. [Google Scholar] [CrossRef]
- Konda, S.K.; Chen, A. Palladium based nanomaterials for enhanced hydrogen spillover and storage. Mater. Today 2016, 19, 100–108. [Google Scholar] [CrossRef]
- Zhou, C.; Szpunar, J.A.; Cui, X. Synthesis of Ni/Graphene nanocomposite for hydrogen storage. ACS Appl. Mater. Interfaces 2016, 8, 15232–15241. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Wang, D.; Zhang, C.; Zhou, X.; Xin, H.; Liu, X.; Cai, M. Spillover enhanced hydrogen uptake of Pt/Pd doped corncob-derived activated carbon with ultra-high surface area at high pressure. Int. J. Hydrogen Energy 2014, 39, 13643–13649. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, J.; Ji, D.; Yuan, A.; Shen, X. Effect of catalyst loading on hydrogen storage capacity of ZIF-8/graphene oxide doped with Pt or Pd via spillover. Micropor. Mesopor. Mater. 2016, 229, 68–75. [Google Scholar] [CrossRef]
- Dibandjo, P.; Zlotea, C.; Gadiou, R.; Matei Ghimbeu, C.; Cuevas, F.; Latroche, M.; Leroy, E.; Vix-Guterl, C. Hydrogen storage in hybrid nanostructured carbon/palladium materials: Influence of particle size and surface chemistry. Int. J. Hydrogen Energy 2013, 38, 952–965. [Google Scholar] [CrossRef]
- Zielinska, B.; Michalkiewicz, B.; Mijowska, E.; Kalenczuk, R.J. Advances in Pd nanoparticle Size decoration of mesoporous carbon spheres for energy application. Nanoscale Res. Lett. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, B.; Michalkiewicz, B.; Chen, X.; Mijowska, E.; Kalenczuk, R.J. Pd supported ordered mesoporous hollow carbon spheres (OMHCS) for hydrogen storage. Chem. Phys. Lett. 2016, 647, 14–19. [Google Scholar] [CrossRef]
- Tew, M.W.; Miller, J.T.; van Bokhoven, J.A. Particle size effect of hydride formation and surface hydrogen adsorption of nanosized palladium catalysts: L3 Edge vs. K Edge X-ray Absorption Spectroscopy. J. Phys. Chem. C 2009, 113, 15140–15147. [Google Scholar] [CrossRef]
- Yamauchi, M.; Ikeda, R.; Kitagawa, H.; Takata, M. Nanosize effects on hydrogen storage in palladium. J. Phys. Chem. C 2008, 112, 3294–3299. [Google Scholar] [CrossRef]
- Baca, M.; Cendrowski, K.; Banach, P.; Michalkiewicz, B.; Mijowska, E.; Kalenczuk, R.J.; Zielinska, B. Effect of Pd loading on hydrogen storage properties of disordered mesoporous hollow carbon spheres. Int. J. Hydrogen Energy 2017, 42, 30461–30469. [Google Scholar] [CrossRef]
- Tsubouchi, N.; Xu, C.; Ohtsuka, Y. Carbon crystallization during high-temperature pyrolysis of coals and the enhancement by calcium. Energy Fuel 2003, 17, 1119–1125. [Google Scholar] [CrossRef]
- Naresh, N.; Wasim, F.G.S.; Ladewig, B.P.; Neergat, M. Removal of surfactant and capping agent from Pd nanocubes (Pd-NCs) using tert-butylamine: Its effect on electrochemical characteristics. J. Mater. Chem. A 2013, 1, 8553–8559. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, Y.; Hu, Y.; Li, C.; Wu, P.; Wei, S.; Cai, C. Pd@Pt core-shell nanostructures with controllable composition synthesized by a microwave method and their enhanced electrocatalytic activity toward oxygen reduction and methanol oxidation. J. Phys. Chem. C 2010, 114. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Pan, C.J.; Liu, J.Y.; Chou, H.L.; Rick, J.; Su, W.N.; Hwang, B.J. Functional palladium tetrapod core of heterogeneous palladium-platinum nanodendrites for enhanced oxygen reduction reaction. J. Power Sources 2014, 251, 393–401. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Ko, A.-R.; Han, S.-B.; Kim, H.-S.; Park, K.-W. Synthesis of octahedral Pt-Pd alloy nanoparticles for improved catalytic activity and stability in methanol electrooxidation. Phys. Chem. Chem. Phys. 2011, 13, 5569–5572. [Google Scholar] [CrossRef] [PubMed]
- Ihsanullah; Al Amer, A.M.; Laoui, T.; Abbas, A.; Al-Aqeeli, N.; Patel, F.; Khraisheh, M.; Atieh, A.M.; Hilal, N. Fabrication and antifouling behaviour of a carbon nanotube membrane. Mater. Des. 2016, 89, 549–558. [Google Scholar] [CrossRef]
- Manasrah, A.D.; Almanassra, I.W.; Marei, N.N.; Al-Mubaiyedh, U.A.; Laoui, T.; Atieh, M.A. Surface modification of carbon nanotubes with copper oxide nanoparticles for heat transfer enhancement of nanofluids. RSC Adv. 2018, 8, 1791–1802. [Google Scholar] [CrossRef] [Green Version]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jia, M.; Gao, H.; Yu, J.; Wang, L.; Zou, Y.; Qin, F.; Zhao, Y. Porous hollow carbon spheres: facile fabrication and excellent supercapacitive properties. Electrochim. Acta 2015, 184, 32–39. [Google Scholar] [CrossRef]
- Sawant, S.Y.; Somani, R.S.; Newalkar, B.L.; Choudary, N.V.; Bajaj, H.C. Synthesis of submicron size hollow carbon spheres by a chemical reduction–solvothermal method using carbon tetrachloride as carbon source. Mater. Lett. 2009, 63, 2339–2342. [Google Scholar] [CrossRef]
- Schneider, P. Adsorption isotherms of microporous-mesoporous solids revisited. Appl. Catal. A 1995, 129, 157–165. [Google Scholar] [CrossRef]
- Dhawana, R.; Goyal, M.; Bhasin, K.K. Influence of metal impregnants on adsorption of dimethylsulfide vapors by activated carbons. Mater. Today 2017, 4, 10515–10519. [Google Scholar] [CrossRef]
- Yang, C.-M.; Lin, H.-A.; Zibrowius, B.; Spliethoff, B.; Schüth, F.; Liou, S.-C.; Chu, M.-W.; Chen, C.-H. Selective surface functionalization and metal deposition in the micropores of mesoporous silica SBA-15. Chem. Mater. 2007, 19, 3205–3211. [Google Scholar] [CrossRef]
- Chen, C.-L.; Li, T.; Cheng, S.; Lin, H.-P.; Bhongale, C.J.; Mou, C.-Y. Direct impregnation method for preparing sulfated zirconia supported on mesoporous silica. Micropor. Mesopor. Mater. 2001, 50, 201–208. [Google Scholar] [CrossRef]
- Zhong, M.; Fu, Z.; Yuan, L.; Zhao, H.; Zhu, J.; He, Y.; Wang, C.; Tang, Y. A solution-phase synthesis method to prepare Pd-doped carbon aerogels for hydrogen storage. RSC Adv. 2015, 5, 20966–20971. [Google Scholar] [CrossRef]
- Zubizarreta, L.; Menéndez, J.A.; Pis, J.J.; Arenillas, A. Improving hydrogen storage in Ni-doped carbon nanospheres. Int. J. Hydrogen Energy 2009, 34, 3070–3076. [Google Scholar] [CrossRef]
- Deeba, M.; Luo, T.; Ramos, J. Palladium-Supported Catalyst Composites. U.S. Patent 8,568,675 B2, 29 October 2013. [Google Scholar]








| Sample | SBET (m2 g−1) | TPV (cm3 g−1) | Vmicro (cm3 g−1) |
|---|---|---|---|
| HCS | 676 | 0.9957 | 0.312 |
| Pd_HCS | 588 | 0.7648 | 0.274 |
| Pt_HCS | 576 | 0.8374 | 0.271 |
| Pt/Pd_HCS | 535 | 0.7046 | 0.248 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baca, M.; Cendrowski, K.; Kukulka, W.; Bazarko, G.; Moszyński, D.; Michalkiewicz, B.; Kalenczuk, R.J.; Zielinska, B. A Comparison of Hydrogen Storage in Pt, Pd and Pt/Pd Alloys Loaded Disordered Mesoporous Hollow Carbon Spheres. Nanomaterials 2018, 8, 639. https://0-doi-org.brum.beds.ac.uk/10.3390/nano8090639
Baca M, Cendrowski K, Kukulka W, Bazarko G, Moszyński D, Michalkiewicz B, Kalenczuk RJ, Zielinska B. A Comparison of Hydrogen Storage in Pt, Pd and Pt/Pd Alloys Loaded Disordered Mesoporous Hollow Carbon Spheres. Nanomaterials. 2018; 8(9):639. https://0-doi-org.brum.beds.ac.uk/10.3390/nano8090639
Chicago/Turabian StyleBaca, Martyna, Krzysztof Cendrowski, Wojciech Kukulka, Grzegorz Bazarko, Dariusz Moszyński, Beata Michalkiewicz, Ryszard J. Kalenczuk, and Beata Zielinska. 2018. "A Comparison of Hydrogen Storage in Pt, Pd and Pt/Pd Alloys Loaded Disordered Mesoporous Hollow Carbon Spheres" Nanomaterials 8, no. 9: 639. https://0-doi-org.brum.beds.ac.uk/10.3390/nano8090639