Combined Effects of Test Media and Dietary Algae on the Toxicity of CuO and ZnO Nanoparticles to Freshwater Microcrustaceans Daphnia magna and Heterocypris incongruens: Food for Thought
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Test Media
2.3. Physico-Chemical Characterisation of Nanoparticle Suspensions
2.4. Test Formats of Bioassays
2.5. Statistical Analysis
3. Results
3.1. Behaviour of CuO and ZnO Nanoparticles in the Test Media
3.1.1. Stability of Nanoparticle Suspensions
3.1.2. Dissolution of CuO and ZnO Nanoparticles in the Test Media
3.2. Toxicity of Cu and Zn Compounds to Daphnia magna and Heterocypris Incongruens
3.2.1. Toxicity of Cu Compounds to D. magna
3.2.2. Toxicity of Zn Compounds to D. magna
3.2.3. Toxicity of Cu and Zn Compounds to H. incongruens
4. Discussion
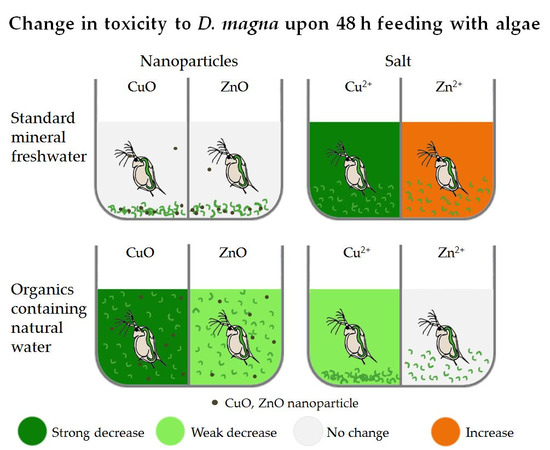
4.1. Combined Effect of the Media and Feeding on D. magna Toxicity Test Results
4.2. Differences between CuO and ZnO Nanoparticle Toxicity to D. magna and H. incongruens
5. Conclusions
- Subchronic (6 day) H. incongruens LC50 for CuO NP was 1.1 mg Cu/L, and 0.22 mg Cu/L for CuSO4, in US EPA mineral water. For both ZnO NP and ZnSO4, the respective 6-day LC50 was 0.36 mg Zn/L. For comparison, upon the addition of dietary algae in mineral medium, 48 h EC50 of CuO and ZnO NP for D. magna was ~2 mg metal/L;
- Compared to standard mineral media, natural freshwater mitigated CuO NP toxicity (4–18-fold) and increased ZnO NP toxicity (3–4-fold) for D. magna. For Cu and Zn salts, the toxicity change followed the same pattern with 3–4-fold mitigation and an increase in natural water. In H. incongruens tests (all including algae), toxicity was mitigated only up to 2-fold (CuO NP and Cu salt) or remained the same (Zn compounds) in natural water;
- Upon the addition of algae to D. magna for 48 h in OECD mineral medium, no toxicity mitigating effect was recorded for CuO NP, possibly due to the sedimentation of algae and NP. CuSO4 48 h EC50, however, decreased 8-fold. For ZnO NP and ZnSO4, the added algae resulted in comparable or even increased (ZnSO4) toxicity;
- Algae in natural freshwater mitigated both CuO NP (5 to >10 fold) and Cu salt (3-fold) toxicity. The toxicity of ZnO was also significantly reduced (4-fold) but Zn salt toxicity remained unchanged.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. Communication (COM(2012) 572 Final) from the Commission to the European Parliament, the Council and the European Economic and Social Committee: Second Regulatory Review on Nanomaterials; European Commission: Brussels, Belgium, 2013. [Google Scholar]
- Holden, P.A.; Gardea-Torresdey, J.L.; Klaessig, F.; Turco, R.F.; Mortimer, M.; Hund-Rinke, K.; Cohen Hubal, E.A.; Avery, D.; Barceló, D.; Behra, R.; et al. Considerations of Environmentally Relevant Test Conditions for Improved Evaluation of Ecological Hazards of Engineered Nanomaterials. Environ. Sci. Technol. 2016, 50, 6124–6145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blinova, I.; Vija, H.; Lukjanova, A.; Muna, M.; Syvertsen-wiig, G.; Kahru, A. Assessment of the hazard of nine (doped) lanthanides-based ceramic oxides to four aquatic species. Sci. Total Environ. 2018, 612, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Garner, K.L.; Suh, S.; Lenihan, H.S.; Keller, A.A. Species sensitivity distributions for engineered nanomaterials. Environ. Sci. Technol. 2015, 49, 5753–5759. [Google Scholar] [CrossRef] [PubMed]
- Juganson, K.; Ivask, A.; Blinova, I.; Mortimer, M.; Kahru, A. NanoE-Tox: New and in-depth database concerning ecotoxicity of nanomaterials. Beilstein J. Nanotechnol. 2015, 6, 1788–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scientific Committee on Emerging and Newly Identified Health Risks. Risk Assessment of Products of Nanotechnologies; European Commission: Brussels, Belgium, 2009. [Google Scholar]
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 2013, 15, 1692. [Google Scholar] [CrossRef]
- European Chemicals Agency. Literature Study on the Uses and Risks of Nanomaterials as Pigments in the European Union; European Chemicals Agency: Helsinki, Finland, 2018. [Google Scholar]
- Garner, K.L.; Suh, S.; Keller, A.A. Assessing the Risk of Engineered Nanomaterials in the Environment: Development and application of the nanoFate model. Environ. Sci. Technol. 2017, 51, 5541–5551. [Google Scholar] [CrossRef] [PubMed]
- Notter, D.A.; Mitrano, D.M.; Nowack, B. Are nanosized or dissolved metals more toxic in the environment? A meta-analysis. Environ. Toxicol. Chem. 2014, 33, 2733–2739. [Google Scholar] [CrossRef] [PubMed]
- Adam, N.; Leroux, F.; Knapen, D.; Bals, S.; Blust, R. The uptake and elimination of ZnO and CuO nanoparticles in Daphnia magna under chronic exposure scenarios. Water Res. 2015, 68, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Adam, N.; Leroux, F.; Knapen, D.; Bals, S.; Blust, R. The uptake of ZnO and CuO nanoparticles in the water-flea Daphnia magna under acute exposure scenarios. Environ. Pollut. 2014, 194, 130–137. [Google Scholar] [CrossRef]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.-C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [PubMed]
- Thurberg, F.P.; Dawson, M.A.; Collier, R.S. Effects of Copper and Cadmium on Osmoregulation and Oxygen-Consumption in 2 Species of Estuarine Crabs. Mar. Biol. 1973, 23, 171–175. [Google Scholar] [CrossRef]
- Erickson, R.J.; Brooke, L.T.; Kahl, M.D.; Vende Venter, F.; Harting, S.L.; Markee, T.P.; Spehar, R.L. Effects of laboratory test conditions on the toxicity of silver to aquatic organisms. Environ. Toxicol. Chem. 1998, 17, 572–578. [Google Scholar] [CrossRef] [Green Version]
- De Schamphelaere, K.A.; Vasconcelos, F.M.; Tack, F.M.G.; Allen, H.E.; Janssen, C.R. Effect of dissolved organic matter source on acute copper toxicity to Daphnia magna. Environ. Toxicol. Chem. 2004, 23, 1248–1255. [Google Scholar] [CrossRef]
- Käkinen, A.; Bondarenko, O.; Ivask, A.; Kahru, A. The effect of composition of different ecotoxicological test media on free and bioavailable copper from CuSO4 and CuO nanoparticles: Comparative evidence from a Cu-selective electrode and a Cu-biosensor. Sensors 2011, 11, 10502–10521. [Google Scholar] [CrossRef] [PubMed]
- Akhil, K.; Sudheer Khan, S. Effect of humic acid on the toxicity of bare and capped ZnO nanoparticles on bacteria, algal and crustacean systems. J. Photochem. Photobiol. B Biol. 2017, 167, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Blinova, I.; Ivask, A.; Heinlaan, M.; Mortimer, M.; Kahru, A. Ecotoxicity of nanoparticles of CuO and ZnO in natural water. Environ. Pollut. 2010, 158, 41–47. [Google Scholar] [CrossRef]
- Cupi, D.; Hartmann, N.B.; Baun, A. The influence of natural organic matter and aging on suspension stability in guideline toxicity testing of silver, zinc oxide, and titanium dioxide nanoparticles with Daphnia magna. Environ. Toxicol. Chem. 2015, 34, 497–506. [Google Scholar] [CrossRef]
- Barata, C.; Baird, D.J.; Markich, S.J. Influence of genetic and environmental factors on the tolerance of Daphnia magna Straus to essential and non-essential metals. Aquat. Toxicol. 1998, 42, 115–137. [Google Scholar] [CrossRef]
- Chen, K.L.; Elimelech, M. Influence of humic acid on the aggregation kinetics of fullerene (C60) nanoparticles in monovalent and divalent electrolyte solutions. J. Colloid Interface Sci. 2007, 309, 126–134. [Google Scholar] [CrossRef]
- Cupi, D.; Hartmann, N.B.; Baun, A. Influence of pH and media composition on suspension stability of silver, zinc oxide, and titanium dioxide nanoparticles and immobilization of Daphnia magna under guideline testing conditions. Ecotoxicol. Environ. Saf. 2016, 127, 144–152. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Water Quality—Determination of Freshwater Sediment Toxicity to Heterocypris incongruens (Crustacea, Ostracoda); ISO 14371 (E); ISO: Geneva, Switzerland, 2012. [Google Scholar]
- Organisation for Economic Co-operation and Development. Test No. 211: Daphnia magna Reproduction Test, OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2012. [Google Scholar]
- Organisation for Economic Co-operation and Development. Test No. 202: Daphnia sp. Acute Immobilisation Test, OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2004. [Google Scholar]
- Zhang, Y.; Jeppesen, E.; Liu, X.; Qin, B.; Shi, K.; Zhou, Y.; Thomaz, S.M.; Deng, J. Global loss of aquatic vegetation in lakes. Earth-Sci. Rev. 2017, 173, 259–265. [Google Scholar] [CrossRef]
- Stevenson, L.M.; Krattenmaker, K.E.; Johnson, E.; Bowers, A.J.; Adeleye, A.S.; McCauley, E.; Nisbet, R.M. Standardized toxicity testing may underestimate ecotoxicity: Environmentally relevant food rations increase the toxicity of silver nanoparticles to Daphnia. Environ. Toxicol. Chem. 2017, 36, 3008–3018. [Google Scholar] [CrossRef]
- MicroBioTests Inc. OSTRACODTOXKIT F “Direct Contact” Toxicity Test for Freshwater Sediments. Standard Operational Procedure. Available online: www.microbiotests.be/SOPs/Ostracodtoxkit F SOP - A5.pdf (accessed on 24 December 2018).
- Toxic Cyanobacteria in Water. A Guide to Their Public Health Consequences, Monitoring, and Management; Chorus, I., Bartram, J., Eds.; St Edmundsbury Press: Suffolk, UK, 1999; ISBN 0419239308. [Google Scholar]
- Belgis, Z.C.; Persoone, G.; Blaise, C. Cyst-based toxicity tests XVI—Sensitivity comparison of the solid phase Heterocypris incongruens microbiotest with the Hyalella azteca and Chironomus riparius contact assays on freshwater sediments from Peninsula Harbour (Ontario, Canada). Chemosphere 2003, 52, 95–101. [Google Scholar] [CrossRef]
- Gondikas, A.P.; Von Der Kammer, F.; Reed, R.B.; Wagner, S.; Ranville, J.F.; Hofmann, T. Release of TiO2 nanoparticles from sunscreens into surface waters: A one-year survey at the old danube recreational lake. Environ. Sci. Technol. 2014, 48, 5415–5422. [Google Scholar] [CrossRef] [PubMed]
- Coll, C.; Notter, D.; Gottschalk, F.; Sun, T.; Som, C.; Nowack, B. Probabilistic environmental risk assessment of five nanomaterials (nano-TiO2, nano-Ag, nano-ZnO, CNT, and fullerenes). Nanotoxicology 2016, 10, 436–444. [Google Scholar] [CrossRef]
- Khangarot, B.S.; Das, S. Acute toxicity of metals and reference toxicants to a freshwater ostracod, Cypris subglobosa Sowerby, 1840 and correlation to EC50 values of other test models. J. Hazard. Mater. 2009, 172, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.M.; Heinlaan, M.; Sihtmäe, M.; Ivask, A.; Kurvet, I.; Joonas, E.; Jemec, A.; Mannerström, M.; Heinonen, T.; Rekulapelly, R.; et al. Multilaboratory evaluation of 15 bioassays for (eco)toxicity screening and hazard ranking of engineered nanomaterials: FP7 project NANOVALID. Nanotoxicology 2016, 10, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Freshwater Organisms, 4th ed.; United States Environmental Protection Agency: Washington, DC, USA, 2002.
- Gustafsson, J.P. Visual MINTEQ Version 3.1. Available online: https://vminteq.lwr.kth.se/download/ (accessed on 24 December 2018).
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Käkinen, A.; Titma, T.; Heinlaan, M.; et al. Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef] [PubMed]
- Blinova, I.; Lukjanova, A.; Muna, M.; Vija, H.; Kahru, A. Evaluation of the potential hazard of lanthanides to freshwater microcrustaceans. Sci. Total Environ. 2018, 642, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Vindimian, E. MSExcel Macro REGTOX_EV7.0.5.xls. Available online: http://www.normalesup.org/~vindimian/en_index.html (accessed on 24 December 2018).
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Grillo, R.; Rosa, A.H.; Fraceto, L.F. Engineered nanoparticles and organic matter: A review of the state-of-the-art. Chemosphere 2014, 119C, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, C.; Hou, J.; Wang, P.; Miao, L.; Lv, B.; Yang, Y. Aggregation, sedimentation, and dissolution of CuO and ZnO nanoparticles in five waters. Environ. Sci. Pollut. Res. 2018, 25, 31240–31249. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.-W.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and Dissolution of 4 nm ZnO Nanoparticles in Aqueous Enviroments: Influence of pH, Ionic Strength, Size, and Adsorption of Umic Acid. Langumir 2011, 27, 6059–6068. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Kim, D.; Hwang, G.; Lee, B.; Eom, I.; Kim, P.J.; Tong, M.; Kim, H. Aggregation and dissolution of ZnO nanoparticles synthesized by different methods: Influence of ionic strength and humic acid. Colloids Surf. A Physicochem. Eng. Asp. 2014, 451, 7–15. [Google Scholar] [CrossRef]
- Conway, J.R.; Adeleye, A.S.; Gardea-Torresdey, J.; Keller, A.A. Aggregation, dissolution, and transformation of copper nanoparticles in natural waters. Environ. Sci. Technol. 2015, 49, 2749–2756. [Google Scholar] [CrossRef]
- Aruoja, V.; Pokhrel, S.; Sihtmäe, M.; Mortimer, M.; Mädler, L.; Kahru, A. Toxicity of 12 metal-based nanoparticles to algae, bacteria and protozoa. Environ. Sci. Nano 2015, 2, 630–644. [Google Scholar] [CrossRef]
- Ji, J.; Long, Z.; Lin, D. Toxicity of oxide nanoparticles to the green algae Chlorella sp. Chem. Eng. J. 2011, 170, 525–530. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, K.; Yang, K.; Lin, D. Heteroagglomeration of oxide nanoparticles with algal cells: Effects of particle type, ionic strength and pH. Environ. Sci. Technol. 2015, 49, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Quik, J.T.K.; Velzeboer, I.; Wouterse, M.; Koelmans, A.A.; van de Meent, D. Heteroaggregation and sedimentation rates for nanomaterials in natural waters. Water Res. 2014, 48, 269–279. [Google Scholar] [CrossRef]
- Lin, D.; Ji, J.; Long, Z.; Yang, K.; Wu, F. The influence of dissolved and surface-bound humic acid on the toxicity of TiO2 nanoparticles to Chlorella sp. Water Res. 2012, 46, 4477–4487. [Google Scholar] [CrossRef] [PubMed]
- Röhder, L.A.; Brandt, T.; Sigg, L.; Behra, R. Influence of agglomeration of cerium oxide nanoparticles and speciation of cerium(III) on short term effects to the green algae Chlamydomonas reinhardtii. Aquat. Toxicol. 2014, 152, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, A.; Eisenstadt, D.; Bar-Gil, A.; Carmely, H.; Einbinder, S.; Gressel, J. Inexpensive non-toxic flocculation of microalgae contradicts theories; overcoming a major hurdle to bulk algal production. Biotechnol. Adv. 2012, 30, 1023–1030. [Google Scholar] [CrossRef]
- Muna, M.; Heinlaan, M.; Blinova, I.; Vija, H.; Kahru, A. Evaluation of the effect of test medium on total Cu body burden of nano CuO-exposed Daphnia magna: A TXRF spectroscopy study. Environ. Pollut. 2017, 1–9. [Google Scholar] [CrossRef]
- Stiff, M.J. The chemical states of copper in polluted fresh water and a scheme of analysis to differentiate them. Water Res. 1971, 5, 585–599. [Google Scholar] [CrossRef]
- Odzak, N.; Kistler, D.; Behra, R.; Sigg, L. Dissolution of metal and metal oxide nanoparticles under natural freshwater conditions. Environ. Chem. 2015, 12, 138–148. [Google Scholar] [CrossRef]
- Heinlaan, M.; Muna, M.; Knöbel, M.; Kistler, D.; Odzak, N.; Kühnel, D.; Müller, J.; Gupta, G.S.; Kumar, A.; Shanker, R.; et al. Natural water as the test medium for Ag and CuO nanoparticle hazard evaluation: An interlaboratory case study. Environ. Pollut. 2016, 216, 689–699. [Google Scholar] [CrossRef]
- Li, M.; Lin, D.; Zhu, L. Effects of water chemistry on the dissolution of ZnO nanoparticles and their toxicity to Escherichia coli. Environ. Pollut. 2013, 173, 97–102. [Google Scholar] [CrossRef]
- Borgmann, U.; Charlton, C.C. Copper complexation and toxicity to Daphnia in natural waters. J. Great Lakes Res. 1984, 10, 393–398. [Google Scholar] [CrossRef]
- Hyne, R.V.; Pablo, F.; Julli, M.; Markich, S.J. Influence of water chemistry on the acute toxicity of copper and zinc to the cladoceran Ceriodaphnia cf dubia. Environ. Toxicol. Chem. 2005, 24, 1667–1675. [Google Scholar] [CrossRef]
- Sevilla, J.B.; Nakajima, F.; Kasuga, I. Comparison of aquatic and dietary exposure of heavy metals Cd, Cu, and Zn to benthic ostracod Heterocypris incongruens. Environ. Toxicol. Chem. 2014, 33, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Kudłak, B.; Wolska, L.; Namieśnik, J. Determination of EC50 toxicity data of selected heavy metals toward Heterocypris incongruens and their comparison to “direct-contact” and microbiotests. Environ. Monit. Assess. 2011, 174, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, K.E.; Christensen, G.M. Effects of Various Metals on Survival, Growth, Reproduction, and Metabolism of Daphnia magna. J. Fish. Res. Board Can. 1972, 29, 1691–1700. [Google Scholar] [CrossRef]
- Xu, H.; Pan, J.; Zhang, H.; Yang, L. Interactions of metal oxide nanoparticles with extracellular polymeric substances (EPS) of algal aggregates in an eutrophic ecosystem. Ecol. Eng. 2016, 94, 464–470. [Google Scholar] [CrossRef]
- Dalai, S.; Iswarya, V.; Bhuvaneshwari, M.; Pakrashi, S.; Chandrasekaran, N.; Mukherjee, A. Different modes of TiO2 uptake by Ceriodaphnia dubia: Relevance to toxicity and bioaccumulation. Aquat. Toxicol. 2014, 152, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Gong, N.; Shao, K.; Li, G.; Sun, Y. Acute and chronic toxicity of nickel oxide nanoparticles to Daphnia magna: The influence of algal enrichment. NanoImpact 2016, 3–4, 104–109. [Google Scholar] [CrossRef]
- Wu, F.; Bortvedt, A.; Harper, B.J.; Crandon, L.E.; Harper, S.L. Uptake and toxicity of CuO nanoparticles to Daphnia magna varies between indirect dietary and direct waterborne exposures. Aquat. Toxicol. 2017, 190, 78–86. [Google Scholar] [CrossRef]
- Seo, J.; Kim, S.; Choi, S.; Kwon, D.; Yoon, T.-H.; Kim, W.-K.; Park, J.-W.; Jung, J. Effects of Physiochemical Properties of Test Media on Nanoparticle Toxicity to Daphnia magna Straus. Bull. Environ. Contam. Toxicol. 2014, 93, 257–262. [Google Scholar] [CrossRef]
- De Schamphelaere, K.A.; Janssen, C.R. Effects of dissolved organic carbon concentration and source, pH, and water hardness on chronic toxicity of copper to Daphnia magna. Environ. Toxicol. Chem. 2004, 23, 1115–1122. [Google Scholar] [CrossRef]
- Bianchini, A.; Wood, C.M. Does sulfide or water hardness protect against chronic silver toxicity in Daphnia magna? A critical assessment of the acute-to-chronic toxicity ratio for silver. Ecotoxicol. Environ. Saf. 2008, 71, 32–40. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Zhang, X.; Chang, Y.; Chen, Y. The impact of ZnO nanoparticle aggregates on the embryonic development of zebrafish (Danio rerio). Nanotechnology 2009, 20, 195103. [Google Scholar] [CrossRef] [PubMed]
- Havel, J.E.; Talbott, B.L. Life history characteristics of the freshwater ostracod Cyprinotus incongruens and their application to toxicity testing. Ecotoxicology 1995, 4, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Shuhaimi-Othman, M.; Yakub, N.; Ramle, N.A.; Abas, A. Toxicity of metals to a freshwater ostracod: Stenocypris major. J. Toxicol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
| Experiment Type | Test Organism | Test Duration | Algae R. subcapitata (Cells/mL) | Test Medium |
|---|---|---|---|---|
| Acute | Daphnia magna | 48 h | no | AFW, two lake waters |
| Acute | Daphnia magna | 48 h | 7.5 × 106 | AFW, two lake waters |
| Subchronic | Heterocypris incongruens | 6 days | 7.5 × 106 | MHW, two lake waters |
| D. magna AFW | H. incongruens MHW | Lake Raku | Lake Ülemiste | |
|---|---|---|---|---|
| pH | 7.8 | 7.6 | 8.3 (0.035) | 8.2 (0.45) |
| Conductivity 25 °C (μS/cm) | 640 1 | 343 2 | 283 (5.7) | 399 (60) |
| Total organic carbon (mg/L) | 0 | 0 | 5.1 (0.21) | 10 (0.45) |
| Total hardness (mg-ekv/L) | 5 | 1.7 | 2.7 (0.10) | 3.9 (0.56) |
| Total phosphorous (mgP/L) | 0 | 0 | 0.035 (0.00071) | 0.030 (0.012) |
| Total nitrogen (mgN/L) | 0 | 0 | 0.62 (0.13) | 1.4 (0.40) |
| Cl− (mg/L) | 73 | 1.9 | 3.4 (0.28) | 11 (2.1) |
| SO42− (mg/L) | 48 | 93 | 22 (0) | 29 (4.5) |
| Ca2+ (mg/L) | 80 | 14 | 44 (2.5) | 66 (11) |
| Mg2+ (mg/L) | 12 | 12 | 4.6 (0.10) | 7.8 (0.50) |
| Na+ (mg/L) | 18 | 26 | 2.7 (0.021) | 6.7 (1.0) |
| Cu2+ (μg/L) | 0 | 0 | 1.0 (0.25) | 0.64 (0.19) |
| Zn2+ (μg/L) | 0 | 0 | 0.66 (0.45) | 0.69 (0.36) |
| MQ | AFW | MHW | Lake Raku | Lake Ülemiste | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incubation Time | 24 h | 48 h | 48 h 1 | 6 Day 1 | 48 h | 6 Day 1 | 48 h | 6 Day 1 | ||||
| Algae | No | No | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| CuO | 6.9 (1.6) | 0.67 2 (0.47) | 1.5 (0.8) | 0.42 | 1.7 | 1.8 | 0.90 2 (0.42) | 1.1 (0.53) | 1.0 | 1.2 2 (0.58) | 1.8 (0.29) | 2.0 |
| CuSO4 | 84 (5.6) | 37 (6.2) | 47 (4.9) | 37 | 40 | 26 | 33 (0.76) | 45 (4.1) | 31 | 32 (0.81) | 42 (1.3) | 37 |
| ZnO | 27 (1.9) | 24 (6.3) | 44 (6.8) | 25 | 55 | 51 | 21 (2.7) | 57 (37) | 83 | 23 (1.5) | 54 (28) | 76 |
| ZnSO4 | 88 (9.1) | 102 (14) | 94 (5.3) | 97 | 97 | 81 | 90 (0.012) | 86 (19) | 97 | 91 (2.0) | 90 (1.3) | 90 |
| D. magna Acute EC50 (48 h) | D. magna Acute EC50 (48 h) with Algae | H. incongruens Subchronic LC50 (6 days) with Algae | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AFW | Lake Raku | Lake Ülemiste | AFW | Lake Raku | Lake Ülemiste | MHW | Lake Raku | Lake Ülemiste | |
| CuO NP | 1.6 * (1.1−3.4) | 6.3 (3.9−13) | 28 (18−53) | 2.0 (1.7−2.2) | 68 (57−80) | >150 | 1.1 (1.1−1.6) | 1.9 (1.4−3.3) | 2.2 (0.77−4.2) |
| CuSO4 | 0.053 * (0.047−0.059) | 0.15 (0.089−0.18) | 0.22 (0.20−0.25) | 0.41 (0.35−0.51) | 0.50 (0.35−0.70) | 0.65 (0.56−0.76) | 0.22 (0.20−0.24) | 0.25 (0.23−0.25) | 0.44 (0.42−0.49) |
| ZnO NP | 1.9 * (1.7−2.2) | 0.50 (0.46−0.58) | 0.71 (0.59−0.97) | 1.7 (1.6−2.2) | 1.9 (1.4−2.6) | 3.1 (2.1−4.5) | 0.36 (0.30−0.49) | 0.51 (0.38−0.62) | 0.65 (0.54−0.70) |
| ZnSO4 | 2.3 * (1.9−2.9) | 0.59 (0.53−0.79) | 0.76 (0.66−0.91) | 1.5 (1.4−1.7) | 0.84 (0.82−0.89) | 1.3 (1.3−1.4) | 0.36 (0.12−0.46) | 0.43 (0.39−0.48) | 0.43 (0.40−0.50) |
| Copper Compounds | Zinc Compounds | ||||||
|---|---|---|---|---|---|---|---|
| AFW | Lake Raku | Lake Ülemiste | AFW | Lake Raku | Lake Ülemiste | ||
| Change in toxicity 1 (EC50 with algae/EC50 no algae) | NP | 1.3 | 11 | >5 | 0.9 | 3.8 | 4.4 |
| salt | 7.7 | 3.3 | 3.0 | 0.65 | 1.4 | 1.7 | |
| Change in metal recovery 2 (no algae/with algae) | NP | 0.45 | 0.82 | 0.67 | 0.55 | 0.37 | 0.43 |
| salt | 0.79 | 0.73 | 0.76 | 1.1 | 1.0 | 1.0 | |
 increase increase  no effect no effect  ≤5 fold decrease ≤5 fold decrease  ≥5 fold decrease ≥5 fold decrease | |||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muna, M.; Blinova, I.; Kahru, A.; Vinković Vrček, I.; Pem, B.; Orupõld, K.; Heinlaan, M. Combined Effects of Test Media and Dietary Algae on the Toxicity of CuO and ZnO Nanoparticles to Freshwater Microcrustaceans Daphnia magna and Heterocypris incongruens: Food for Thought. Nanomaterials 2019, 9, 23. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9010023
Muna M, Blinova I, Kahru A, Vinković Vrček I, Pem B, Orupõld K, Heinlaan M. Combined Effects of Test Media and Dietary Algae on the Toxicity of CuO and ZnO Nanoparticles to Freshwater Microcrustaceans Daphnia magna and Heterocypris incongruens: Food for Thought. Nanomaterials. 2019; 9(1):23. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9010023
Chicago/Turabian StyleMuna, Marge, Irina Blinova, Anne Kahru, Ivana Vinković Vrček, Barbara Pem, Kaja Orupõld, and Margit Heinlaan. 2019. "Combined Effects of Test Media and Dietary Algae on the Toxicity of CuO and ZnO Nanoparticles to Freshwater Microcrustaceans Daphnia magna and Heterocypris incongruens: Food for Thought" Nanomaterials 9, no. 1: 23. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9010023