Integrating TiO2/SiO2 into Electrospun Carbon Nanofibers towards Superior Lithium Storage Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials Fabrication
2.2. Structural Characterization
2.3. Electrochemical Measurements
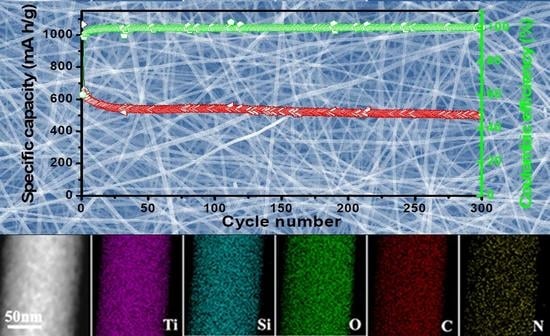
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chan, C.K.; Peng, H.L.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Deng, D.; Kim, M.G.; Lee, J.Y.; Cho, J. Green energy storage materials: Nanostructured TiO2 and Sn-based anodes for lithium-ion batteries. Energy Environ. Sci. 2009, 2, 818–837. [Google Scholar] [CrossRef]
- Yu, Y.; Gu, L.; Wang, C.L.; Dhanabalan, A.; van Aken, P.A.; Maier, J. Encapsulation of Sn@carbon Nanoparticles in Bamboo-like Hollow Carbon Nanofibers as an Anode Material in Lithium-Based Batteries. Angew. Chem. Int. Ed. 2009, 48, 6485–6489. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Zhou, X.; Wu, J.; Yao, X.; Liu, Z. Scalable Synthesis of TiO2/Graphene Nanostructured Composite with High-Rate Performance for Lithium Ion Batteries. ACS Nano 2012, 6, 11035–11043. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Song, T.; Lee, E.-K.; Devadoss, A.; Jeon, Y.; Ha, J.; Chung, Y.-C.; Choi, Y.-M.; Jung, Y.-G.; Paik, U. Dominant Factors Governing the Rate Capability of a TiO2 Nanotube Anode for High Power Lithium Ion Batteries. ACS Nano 2012, 6, 8308–8315. [Google Scholar] [CrossRef]
- Shin, J.Y.; Samuelis, D.; Maier, J. Sustained Lithium-Storage Performance of Hierarchical, Nanoporous Anatase TiO2 at High Rates: Emphasis on Interfacial Storage Phenomena. Adv. Funct. Mater. 2011, 21, 3464–3472. [Google Scholar] [CrossRef]
- Lu, Z.G.; Yip, C.T.; Wang, L.P.; Huang, H.T.; Zhou, L.M. Hydrogenated TiO2 Nanotube Arrays as High-Rate Anodes for Lithium-Ion Microbatteries. Chempluschem 2012, 77, 991–1000. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhao, Y.Y.; Molhave, K.; Sun, H.Y. Engineering the Surface/Interface Structures of Titanium Dioxide Micro and Nano Architectures towards Environmental and Electrochemical Applications. Nanomaterials 2017, 7, 382. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.; Jaroniec, M. Anatase TiO2 with Dominant High-Energy {001} Facets: Synthesis, Properties, and Applications. Chem. Mater. 2011, 23, 4085–4093. [Google Scholar] [CrossRef]
- Chen, J.S.; Archer, L.A.; Lou, X.W. SnO2 hollow structures and TiO2 nanosheets for lithium-ion batteries. J. Mater. Chem. 2011, 21, 9912–9924. [Google Scholar] [CrossRef]
- Zuniga, L.; Agubra, V.; Flores, D.; Campos, H.; Villareal, J.; Alcoutlabi, M. Multichannel hollow structure for improved electrochemical performance of TiO2/Carbon composite nanofibers as anodes for lithium ion batteries. J. Alloys Compd. 2016, 686, 733–743. [Google Scholar] [CrossRef]
- Gao, B.; Sinha, S.; Fleming, L.; Zhou, O. Alloy formation in nanostructured silicon. Adv. Mater. 2001, 13, 816–819. [Google Scholar] [CrossRef]
- Sim, S.; Oh, P.; Park, S.; Cho, J. Critical Thickness of SiO2 Coating Layer on Core@Shell Bulk@Nanowire Si Anode Materials for Li-Ion Batteries. Adv. Mater. 2013, 25, 4498–4503. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, B.; Fu, Z.W. Lithium electrochemistry of SiO2 thin film electrode for lithium-ion batteries. Appl. Surf. Sci. 2008, 254, 3774–3779. [Google Scholar] [CrossRef]
- Guo, B.; Shu, J.; Wang, Z.; Yang, H.; Shi, L.; Liu, Y.; Chen, L. Electrochemical reduction of nano-SiO2 in hard carbon as anode material for lithium ion batteries. Electrochem. Commun. 2008, 10, 1876–1878. [Google Scholar] [CrossRef]
- Nan, D.; Wang, J.-G.; Huang, Z.-H.; Wang, L.; Shen, W.; Kang, F. Highly porous carbon nanofibers from electrospun polyimide/SiO2 hybrids as an improved anode for lithium-ion batteries. Electrochem. Commun. 2013, 34, 52–55. [Google Scholar] [CrossRef]
- Chang, W.S.; Park, C.M.; Kim, J.H.; Kim, Y.U.; Jeong, G.; Sohn, H.J. Quartz (SiO2): A new energy storage anode material for Li-ion batteries. Energy Environ. Sci. 2012, 5, 6895–6899. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, J.; Xue, L.; Huang, T.; Yu, A. Carbon-coated SiO2 nanoparticles as anode material for lithium ion batteries. J. Power Sources 2011, 196, 10240–10243. [Google Scholar] [CrossRef]
- Favors, Z.; Wang, W.; Bay, H.H.; George, A.; Ozkan, M.; Ozkan, C.S. Stable Cycling of SiO2 Nanotubes as High-Performance Anodes for Lithium-Ion Batteries. Sci. Rep. 2014, 4, 4605. [Google Scholar] [CrossRef]
- Yan, N.; Wang, F.; Zhong, H.; Li, Y.; Wang, Y.; Hu, L.; Chen, Q. Hollow Porous SiO2 Nanocubes Towards High-performance Anodes for Lithium-ion Batteries. Sci. Rep. 2013, 3, 1568. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, M.; Liu, D.; Gunawardhana, N.; Yoshio, M.; Nakashima, K. Synthesis, characterization and application for lithium-ion rechargeable batteries of hollow silica nanospheres. J. Mater. Chem. 2011, 21, 13881–13888. [Google Scholar] [CrossRef]
- Dierkes, W.; Blume, A. Silica Reinforcement. In Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–7. [Google Scholar]
- Wang, H.; Yang, X.; Wu, Q.; Zhang, Q.; Chen, H.; Jing, H.; Wang, J.; Mi, S.-B.; Rogach, A.L.; Niu, C. Encapsulating Silica/Antimony into Porous Electrospun Carbon Nanofibers with Robust Structure Stability for High-Efficiency Lithium Storage. ACS Nano 2018, 12, 3406–3416. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, Q.; Cao, D.; Lu, X.; Wang, J.; Leung, M.K.H.; Cheng, S.; Lu, L.; Niu, C. Synthesis of SnSb-embedded carbon-silica fibers via electrospinning: Effect of TEOS on structural evolutions and electrochemical properties. Mater. Today Energy 2016, 1–2, 24–32. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Shen, D.; Zhao, D.; Wang, G. Graphitic Carbon Conformal Coating of Mesoporous TiO2 Hollow Spheres for High-Performance Lithium Ion Battery Anodes. J. Am. Chem. Soc. 2015, 137, 13161–13166. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yu, X.-Y.; Zhou, H.; Wu, H.B.; Ding, S.; Lou, X.W. Bowl-like SnO2@Carbon Hollow Particles as an Advanced Anode Material for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2014, 53, 12803–12807. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, H.; Niu, C.; Rogach, A.L. Ternary Sn-Ti-O Based Nanostructures as Anodes for Lithium Ion Batteries. Small 2015, 11, 1364–1383. [Google Scholar] [CrossRef]
- Lu, X.; Wang, P.; Liu, K.; Niu, C.; Wang, H. Encapsulating nanoparticulate Sb/MoOx into porous carbon nanofibers via electrospinning for efficient lithium storage. Chem. Eng. J. 2018, 336, 701–709. [Google Scholar] [CrossRef]
- Wang, H.; Lu, X.; Li, L.; Li, B.; Cao, D.; Wu, Q.; Li, Z.; Yang, G.; Guo, B.; Niu, C. Synthesis of SnO2 versus Sn crystals within N-doped porous carbon nanofibers via electrospinning towards high-performance lithium ion batteries. Nanoscale 2016, 8, 7595–7603. [Google Scholar] [CrossRef]
- Xu, H.; Hu, X.; Luo, W.; Sun, Y.; Yang, Z.; Hu, C.; Huang, Y. Electrospun Conformal Li4Ti5O12/C Fibers for High-Rate Lithium-Ion Batteries. ChemElectroChem 2014, 1, 611–616. [Google Scholar] [CrossRef]
- Li, Y.; Lu, X.; Wang, H.; Xie, C.; Yang, G.; Niu, C. Growth of Ultrafine SnO2 Nanoparticles within Multiwall Carbon Nanotube Networks: Non-Solution Synthesis and Excellent Electrochemical Properties as Anodes for Lithium Ion Batteries. Electrochim. Acta 2015, 178, 778–785. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef]
- Wang, H.; Xi, L.; Tucek, J.; Ma, C.; Yang, G.; Leung, M.K.H.; Zboril, R.; Niu, C.; Rogach, A.L. Synthesis and Characterization of Tin Titanate Nanotubes: Precursors for Nanoparticulate Sn-Doped TiO2 Anodes with Synergistically Improved Electrochemical Performance. ChemElectroChem 2014, 1, 1563–1569. [Google Scholar] [CrossRef]
- He, L.; Wang, C.; Yao, X.; Ma, R.; Wang, H.; Chen, P.; Zhang, K. Synthesis of carbon nanotube/mesoporous TiO2 coaxial nanocables with enhanced lithium ion battery performance. Carbon 2014, 75, 345–352. [Google Scholar] [CrossRef]
- Yang, Z.X.; Du, G.D.; Meng, Q.; Guo, Z.P.; Yu, X.B.; Chen, Z.X.; Guo, T.L.; Zeng, R. Synthesis of uniform TiO2@carbon composite nanofibers as anode for lithium ion batteries with enhanced electrochemical performance. J. Mater. Chem. 2012, 22, 5848–5854. [Google Scholar] [CrossRef]
- Zhang, X.; Kumar, P.S.; Aravindan, V.; Liu, H.H.; Sundaramurthy, J.; Mhaisalkar, S.G.; Duong, H.M.; Ramakrishna, S.; Madhavi, S. Electrospun TiO2-Graphene Composite Nanofibers as a Highly Durable Insertion Anode for Lithium Ion Batteries. J. Phys. Chem. C 2012, 116, 14780–14788. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Xie, S.; Liu, W.; Niu, C. Template synthesis of graphitic hollow carbon nanoballs as supports for SnOx nanoparticles towards enhanced lithium storage performance. Nanoscale 2018, 10, 6159–6167. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V.R. Metal Oxides and Oxysalts as Anode Materials for Li Ion Batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, J.; Wang, Z.; Wang, H.; Zhi, C.; Yu Denis, Y.W.; Rogach Andrey, L. Carbon-Supported Nickel Selenide Hollow Nanowires as Advanced Anode Materials for Sodium-Ion Batteries. Small 2017, 14, 1702669. [Google Scholar] [CrossRef]
- Shi, X.; Liu, S.; Tang, B.; Lin, X.; Li, A.; Chen, X.; Zhou, J.; Ma, Z.; Song, H. SnO2/TiO2 nanocomposites embedded in porous carbon as a superior anode material for lithium-ion batteries. Chem. Eng. J. 2017, 330, 453–461. [Google Scholar] [CrossRef]
- Wang, N.; Bai, Z.; Qian, Y.; Yang, J. Double-Walled Sb@TiO2−x Nanotubes as a Superior High-Rate and Ultralong-Lifespan Anode Material for Na-Ion and Li-Ion Batteries. Adv. Mater. 2016, 28, 4126–4133. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Yao, T.; Xie, S.; She, Y.; Wang, H. Integrating TiO2/SiO2 into Electrospun Carbon Nanofibers towards Superior Lithium Storage Performance. Nanomaterials 2019, 9, 68. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9010068
Liu W, Yao T, Xie S, She Y, Wang H. Integrating TiO2/SiO2 into Electrospun Carbon Nanofibers towards Superior Lithium Storage Performance. Nanomaterials. 2019; 9(1):68. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9010068
Chicago/Turabian StyleLiu, Wenxing, Tianhao Yao, Sanmu Xie, Yiyi She, and Hongkang Wang. 2019. "Integrating TiO2/SiO2 into Electrospun Carbon Nanofibers towards Superior Lithium Storage Performance" Nanomaterials 9, no. 1: 68. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9010068