Removal of Mercury (II) by EDTA-Functionalized Magnetic CoFe2O4@SiO2 Nanomaterial with Core-Shell Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CoFe2O4@SiO2
2.3. Preparation of CoFe2O4@SiO2-EDTA
2.4. Sample Characterization
2.5. Batch Adsorption Experiments
3. Results and Discussion
3.1. Characterizations
3.2. Adsorption Performance
3.2.1. Effect of EDTA Addition Amount
3.2.2. Effect of pH
3.2.3. Effect of Dosage
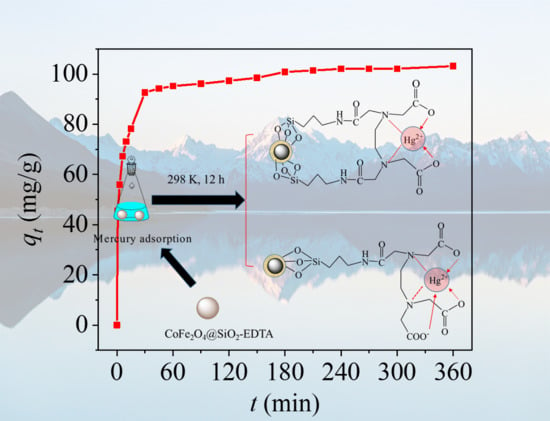
3.3. Adsorption Kinetics
3.4. Adsorption Isotherms
3.5. Thermodynamics
3.6. Reusability
3.7. Mechanism Speculation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, Y.; Zhou, B.; Li, N.; Li, Y.; Han, R.; Qi, J.; Lu, X.; Li, S.; Feng, C.; Liang, S. Spatial-temporal analysis of selected industrial aquatic heavy metal pollution in China. J. Clean. Prod. 2019, 238, 117944. [Google Scholar] [CrossRef]
- Mehmood, A.; Mirza, M.A.; Choudhary, M.A.; Kim, K.-H.; Raza, W.; Raza, N.; Lee, S.S.; Zhang, M.; Lee, J.-H.; Sarfraz, M. Spatial distribution of heavy metals in crops in a wastewater irrigated zone and health risk assessment. Environ. Res. 2019, 168, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Cadar, O.; Miclean, M.; Cadar, S.; Tanaselia, C.; Senila, L.; Senila, M. Assessment of heavy metals in cows milk in rodnei mountains area, Romania. Environ. Eng. Manag. J. 2015, 14, 2523–2528. [Google Scholar] [CrossRef]
- Zhu, H.; Shen, Y.; Wang, Q.; Chen, K.; Wang, X.; Zhang, G.; Yang, J.; Guo, Y.; Bai, R. Highly promoted removal of Hg (ii) with magnetic CoFe2O4@SiO2 core–shell nanoparticles modified by thiol groups. RSC Adv. 2017, 7, 39204–39215. [Google Scholar] [CrossRef]
- Nemati, Y.; Zahedi, P.; Baghdadi, M.; Ramezani, S. Microfluidics combined with ionic gelation method for production of nanoparticles based on thiol-functionalized chitosan to adsorb Hg (II) from aqueous solutions. J. Environ. Manag. 2019, 238, 166–177. [Google Scholar] [CrossRef]
- Ballav, N.; Das, R.; Giri, S.; Muliwa, A.M.; Pillay, K.; Maity, A. l-cysteine doped polypyrrole (PPy@L-Cyst): A super adsorbent for the rapid removal of Hg2+ and efficient catalytic activity of the spent adsorbent for reuse. Chem. Eng. J. 2018, 345, 621–630. [Google Scholar] [CrossRef]
- Saleh, T.A.; Sarı, A.; Tuzen, M. Optimization of parameters with experimental design for the adsorption of mercury using polyethylenimine modified-activated carbon. J. Environ. Chem. Eng. 2017, 5, 1079–1088. [Google Scholar] [CrossRef]
- Danmaliki, G.I.; Saleh, T.A. Effects of bimetallic Ce/Fe nanoparticles on the desulfurization of thiophenes using activated carbon. Chem. Eng. J. 2017, 307, 914–927. [Google Scholar] [CrossRef]
- Kazemi, F.; Younesi, H.; Ghoreyshi, A.A.; Bahramifar, N.; Heidari, A. Thiol-incorporated activated carbon derived from fir wood sawdust as an efficient adsorbent for the removal of mercury ion: Batch and fixed-bed column studies. Process. Saf. Environ. Prot. 2016, 100, 22–35. [Google Scholar] [CrossRef]
- Huang, Y.; Du, J.R.; Zhang, Y.; Lawless, D.; Feng, X. Removal of mercury (II) from wastewater by polyvinylamine-enhanced ultrafiltration. Sep. Purif. Technol. 2015, 154, 1–10. [Google Scholar] [CrossRef]
- Lanas, S.G.; Valiente, M.; Tolazzi, M.; Melchior, A. Thermodynamics of Hg2+ and Ag+ adsorption by 3-mercaptopropionic acid-functionalized superparamagnetic iron oxide nanoparticles. J. Therm. Anal. Calorim. 2018, 3, 1153–1162. [Google Scholar] [CrossRef]
- Gai, K.; Avellan, A.; Hoelen, T.P.; Lopez-Linares, F.; Hatakeyama, E.S.; Lowry, G.V. Impact of mercury speciation on its removal from water by activated carbon and organoclay. Water Res. 2019, 157, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lu, M.; Huang, B.; Wang, D.; Wang, G.; Zhou, L. Decoration of defective MoS2 nanosheets with Fe3O4 nanoparticles as superior magnetic adsorbent for highly selective and efficient mercury ions (Hg2+) removal. J. Alloy. Compd. 2018, 737, 113–121. [Google Scholar] [CrossRef]
- Zhuo, W.; Xu, H.; Huang, R.; Zhou, J.; Tong, Z.; Xie, H.; Zhang, X. A chelating polymer resin: Synthesis, characterization, adsorption and desorption performance for removal of Hg(II) from aqueous solution. J. Iran. Chem. Soc. 2017, 14, 2557–2566. [Google Scholar] [CrossRef]
- Zhuang, S.T.; Yin, Y.N.; Wang, J. Simultaneous detection and removal of cobalt ions from aqueous solution by modified chitosan beads. Int. J. Environ. Sci. Technol. 2017, 15, 385–394. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, H.; Wang, Q.; Wang, J.; Cheng, J.; Guo, Y.; Zhou, X.; Bai, R. Adsorption of mercury(ii) with an Fe3O4 magnetic polypyrrole–graphene oxide nanocomposite. RSC Adv. 2017, 7, 18466–18479. [Google Scholar] [CrossRef]
- Gomes, E.C.; De Sousa, A.F.; Vasconcelos, P.H.; Melo, D.Q.; Diógenes, I.C.; De Sousa, E.H.; Nascimento, R.F.D.; Gil, R.A.S.; Longhinotti, E. Synthesis of bifunctional mesoporous silica spheres as potential adsorbent for ions in solution. Chem. Eng. J. 2013, 214, 27–33. [Google Scholar] [CrossRef]
- Zhou, L.; Zou, H.; Wang, Y.; Liu, Z.; Huang, Z.; Luo, T.; Adesina, A.A. Adsorption of uranium(VI) from aqueous solution using phosphonic acid-functionalized silica magnetic microspheres. J. Radioanal. Nucl. Chem. 2016, 310, 1155–1163. [Google Scholar] [CrossRef]
- Jiaqin, D.; Xiaodong, L.; Yunguo, L.; Guangming, Z.; Jie, L.; Biao, S.; Xue, W. Alginate-modified biochar derived from Ca(II)-impregnated biomass: Excellent anti-interference ability for Pb(II) removal. Ecotoxicol. Environ. Saf. 2018, 165, 211–218. [Google Scholar]
- Yang, L.; Wei, Z.; Zhong, W.; Cui, J.; Wei, W. Modifying hydroxyapatite nanoparticles with humic acid for highly efficient removal of Cu(II) from aqueous solution. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 490, 9–21. [Google Scholar] [CrossRef]
- Guan, X.; Yan, S.; Zeng, Q.; Xu, Z.; Chen, Y.; Fan, H. Polyacrylic acid-grafted magnetite nanoparticles for remediation of Pb(II)-contained water. Fibers Polym. 2016, 17, 1131–1139. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, W.; Xia, M.; Zhou, W.; Wan, Q.; Peng, K.; Zou, B. Synthesis of poly(acrylic acid) coated-Fe3O4 superparamagnetic nano-composites and their fast removal of dye from aqueous solution. J. Nanosci. Nanotechnol. 2013, 13, 4627–4633. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Xu, H.; Zhang, F.; Zhu, X.; Li, X. Preparation and adsorption properties of nano magnetite silica gel for methylene blue from aqueous solution. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 546, 244–253. [Google Scholar] [CrossRef]
- Zhao, L.; Dudek, J.; Polkowska-Motrenko, H.; Chmielewski, A.G. A magnetic nanosorbent for cesium removal in aqueous solutions. Radiochim. Acta 2016, 104, 6. [Google Scholar] [CrossRef]
- Ivanets, A.I.; Prozorovich, V.G.; Roshchina, M.Y.; Srivastava, V.; Sillanpää, M. Unusual behavior of MgFe2O4 during regeneration: Desorption versus specific adsorption. Water Sci. Technol. 2019, in press. [Google Scholar] [CrossRef]
- Ivanets, A.; Roshchina, M.; Srivastava, V.; Prozorovich, V.; Dontsova, T.; Nahirniak, S.; Pankov, V.; Hosseini-Bandegharaei, A.; Nguyen Tran, H.; Sillanpää, M. Effect of metal ions adsorption on the efficiency of methylene blue degradation onto MgFe2O4 as Fenton-like catalysts. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 571, 17–26. [Google Scholar] [CrossRef]
- Ivanets, A.I.; Srivastava, V.; Roshchina, M.Y.; Sillanpää, M.; Prozorovich, V.G.; Pankov, V.V. Magnesium ferrite nanoparticles as a magnetic sorbent for the removal of Mn2+, Co2+, Ni2+ and Cu2+ from aqueous solution. Ceram. Int. 2018, 8, 9097–9104. [Google Scholar] [CrossRef]
- Makarchuk, O.; Dontsova, T.; Perekos, A.; Skoblik, A.; Svystunov, Y. Magnetic Mineral Nanocomposite Sorbents for Wastewater Treatment. J. Nanomater. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Zhang, S.; Niu, H.; Cai, Y.; Zhao, X.; Shi, Y. Arsenite and arsenate adsorption on coprecipitated bimetal oxide magnetic nanomaterials: MnFe2O4 and CoFe2O4. Chem. Eng. J. 2010, 158, 599–607. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Cadar, O.; Mesaros, A.; Borodi, G. Sol-gel synthesis of CoFe2O4:SiO2 nanocomposites–insights into the thermal decomposition process of precursors. J. Anal. Appl. Pyrolysis 2017, 125, 169–177. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Cadar, O. Preparation of CoFe2O4/SiO2 Nanocomposites at Low Temperatures Using Short Chain Diols. J. Chem. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Repo, E.; Warchoł, J.K.; Bhatnagar, A.; Mudhoo, A.; Sillanpää, M. Aminopolycarboxylic acid functionalized adsorbents for heavy metals removal from water. Water Res. 2013, 47, 4812–4832. [Google Scholar] [CrossRef] [PubMed]
- Born, T.; Kontoghiorghe, C.N.; Spyrou, A.; Kolnagou, A.; Kontoghiorghes, G.J. EDTA chelation reappraisal following new clinical trials and regular use in millions of patients: Review of preliminary findings and risk/benefit assessment. Toxicol. Mechanisms Methods 2013, 1, 11–17. [Google Scholar] [CrossRef]
- Ravi, S.; Zhang, S.; Lee, Y.-R.; Kang, K.-K.; Kim, J.-M.; Ahn, J.-W.; Ahn, W.-S. EDTA-functionalized KCC-1 and KIT-6 mesoporous silicas for Nd3+ ion recovery from aqueous solutions. J. Ind. Eng. Chem. 2018, 67, 210–218. [Google Scholar] [CrossRef]
- Viltužnik, B.; Košak, A.; Zub, Y.L.; Lobnik, A. Removal of Pb(II) ions from aqueous systems using thiol-functionalized cobalt-ferrite magnetic nanoparticles. J. Sol-Gel Sci. Technol. 2013, 68, 365–373. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Zhao, Y.; Xia, K.; Guo, Y.; Qu, Z.; Bai, R. A Mild and Facile Synthesis of Amino Functionalized CoFe2O4@SiO2 for Hg(II) Removal. Nanomaterials 2018, 8, 673. [Google Scholar] [CrossRef]
- Tan, Z.; Peng, H.; Liu, H.; Wang, L.; Chen, J.; Lü, X. Facile preparation of EDTA-functionalized chitosan magnetic adsorbent for removal of Pb(II). J. Appl. Polym. Sci. 2015, 132, 32. [Google Scholar] [CrossRef]
- Zou, Z.; Shi, Z.; Deng, L. Highly efficient removal of Cu(ii) from aqueous solution using a novel magnetic EDTA functionalized CoFe2O4. RSC Adv. 2017, 7, 5195–5205. [Google Scholar] [CrossRef]
- Li, M.; Li, M.-Y.; Feng, C.-G.; Zeng, Q.-X. Preparation and characterization of multi-carboxyl- functionalized silica gel for removal of Cu(II), Cd(II), Ni(II) and Zn(II) from aqueous solution. Appl. Surf. Sci. 2014, 314, 1063–1069. [Google Scholar] [CrossRef]
- Xu, M.; Li, J.; Yan, Y.; Zhao, X.; Yan, J.; Zhang, Y.; Lai, B.; Chen, X.; Song, L. Catalytic degradation of sulfamethoxazole through peroxymonosulfate activated with expanded graphite loaded CoFe2O4 particles. Chem. Eng. J. 2019, 369, 403–413. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, S.; Ding, J.; Deng, Z.; Guo, L.; Zhong, Q. Enhanced catalytic ozonation for NOx removal with CuFe2O4 nanoparticles and mechanism analysis. J. Mol. Catal. A Chem. 2016, 424, 153–161. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Jiang, Z.; Zhang, Y.; Wen, T.; Fang, M.; Tan, X.; Alsaedi, A.; Hayat, T.; Wang, X. Selective Immobilization of Highly Valent Radionuclides by Carboxyl Functionalized Mesoporous Silica Microspheres: Batch, XPS, and EXAFS Analyses. ACS Sustain. Chem. Eng. 2018, 6, 15644–15652. [Google Scholar] [CrossRef]
- Guo, S.; Dan, Z.; Duan, N.; Chen, G.; Gao, W.; Zhao, W. Zn(II), Pb(II), and Cd(II) adsorption from aqueous solution by magnetic silica gel: Preparation, characterization, and adsorption. Environ. Sci. Pollut. Res. 2018, 25, 30938–30948. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lou, Z.; Sun, Y.; Zhou, X.; Baig, S.A.; Xu, X. Influence of complexing agent on the removal of Pb(II) from aqueous solutions by modified mesoporous SiO2. Microporous Mesoporous Mater. 2017, 246, 1–13. [Google Scholar] [CrossRef]
- Huang, J.; Ye, M.; Qu, Y.; Chu, L.; Chen, R.; He, Q.; Xu, D. Pb(II) removal from aqueous media by EDTA-modified mesoporous silica SBA-15. J. Colloid Interface Sci. 2012, 385, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Repo, E.; Warchol, J.K.; Kurniawan, T.A.; Sillanpää, M.E.T. Adsorption of Co(II) and Ni(II) by EDTA- and/or DTPA-modified chitosan: Kinetic and equilibrium modeling. Chem. Eng. J. 2010, 161, 73–82. [Google Scholar] [CrossRef]
- Liu, W.-J.; Zeng, F.-X.; Jiang, H.; Zhang, X.-S. Adsorption of lead (Pb) from aqueous solution with Typha angustifolia biomass modified by SOCl2 activated EDTA. Chem. Eng. J. 2011, 170, 21–28. [Google Scholar] [CrossRef]
- Wu, D.; Hu, L.; Wang, Y.; Wei, Q.; Yan, L.; Yan, T.; Li, Y.; Du, B. EDTA modified beta-cyclodextrin/chitosan for rapid removal of Pb(II) and acid red from aqueous solution. J. Colloid Interface Sci. 2018, 523, 56–64. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, N.; Liu, S.; Zheng, C.; Wang, X.; Huang, T.; Guo, Y.; Bai, R. Thiol functionalization of short channel SBA-15 through a safe, mild and facile method and application for the removal of mercury (II). J. Environ. Chem. Eng. 2018, 6, 5420–5433. [Google Scholar] [CrossRef]
- Davoodi, S.M.; Taheran, M.; Brar, S.K.; Galvez-Cloutier, R.; Martel, R. Hydrophobic dolomite sorbent for oil spill clean-ups: Kinetic modeling and isotherm study. Fuel 2019, 251, 57–72. [Google Scholar] [CrossRef]
- Cui, H.; Qian, Y.; Li, Q.; Wei, Z.; Zhai, J. Fast removal of Hg(II) ions from aqueous solution by amine-modified attapulgite. Appl. Clay Sci. 2013, 72, 84–90. [Google Scholar] [CrossRef]
- Maia, L.F.O.; Hott, R.C.; Ladeira, P.C.C.; Batista, B.L.; Andrade, T.G.; Santos, M.S.; Faria, M.C.S.; Oliveira, L.C.A.; Monteiro, D.S.; Pereira, M.C.; et al. Simple synthesis and characterization of l-Cystine functionalized delta-FeOOH for highly efficient Hg(II) removal from contamined water and mining waste. Chemosphere 2019, 215, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sui, J.; Li, J.; Tang, Y.; Cai, W. Efficient removal of heavy metal ions by thiol-functionalized superparamagnetic carbon nanotubes. Chem. Eng. J. 2012, 210, 45–52. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, X.; Zhang, Z.; Shen, Y.; Chen, K.; Guo, Y.; Zhou, X.; Bai, R. Synthesis of flower-like Bi2O4/ZnO heterojunction and mechanism of enhanced photodegradation for organic contaminants under visible light. Res. Chem. Intermed. 2018, 44, 6569–6590. [Google Scholar] [CrossRef]
- Plaza, J.; Viera, M.; Donati, E.; Guibal, E. Biosorption of mercury by Macrocystis pyrifera and Undaria pinnatifida: Influence of zinc, cadmium and nickel. J. Environ. Sci. 2011, 23, 1778–1786. [Google Scholar] [CrossRef]
- Takagai, Y.; Shibata, A.; Kiyokawa, S.; Takase, T. Synthesis and evaluation of different thio-modified cellulose resins for the removal of mercury (II) ion from highly acidic aqueous solutions. J. Colloid Interface Sci. 2011, 353, 593–597. [Google Scholar] [CrossRef]
- Hutson, N.D.; Attwood, B.C.; Scheckel, K.G. XAS and XPS Characterization of Mercury Binding on Brominated Activated Carbon. Environ. Sci. Technol. 2007, 41, 1747–1752. [Google Scholar] [CrossRef]
- Mir, A.A.; Amooey, A.A.; Ghasemi, S. Adsorption of direct yellow 12 from aqueous solutions by an iron oxide-gelatin nanoadsorbent; kinetic, isotherm and mechanism analysis. J. Clean. Prod. 2018, 170, 570–580. [Google Scholar] [CrossRef]
- Fallah, Z.; Isfahani, H.N.; Tajbakhsh, M. Cyclodextrin-triazole-titanium based nanocomposite: Preparation, characterization and adsorption behavior investigation. Process. Saf. Environ. Prot. 2019, 124, 251–265. [Google Scholar] [CrossRef]


















| Samples | BET Values (m2/g) | Total Pore Volumes (cm3/g) | Pore Diameters (nm) |
|---|---|---|---|
| CoFe2O4 | 91.85 | 0.176 | 7.67 |
| CoFe2O4@SiO2 | 83.02 | 0.160 | 7.73 |
| CoFe2O4@SiO2-EDTA | 20.09 | 0.065 | 13.02 |
| Pseudo-First-Order | Pseudo-Second-Order | |||||
| qe,exp | qe,cal | k1 | R2 | qe,cal | k2 | R2 |
| 103.13 | 29.80 | 0.013 | 0.925 | 103.62 | 0.009 | 0.999 |
| Intra-Particle Diffusion | ||||||
| kd1 | C1 | R2 | kd2 | C2 | R2 | |
| 11.507 | 35.89 | 0.963 | 0.807 | 88.77 | 0.962 | |
| T (K) | Langmuir Model | Freundlich Model | |||||
| Qm (mg/g) | KL (L/mg) | R2 | RL | 1/n | KF | R2 | |
| 298 | 142.85 | 0.2811 | 0.999 | 0.066 | 0.258 | 54.04 | 0.957 |
| 308 | 138.12 | 0.2491 | 0.996 | 0.074 | 0.256 | 51.39 | 0.968 |
| 318 | 111.23 | 0.2454 | 0.998 | 0.075 | 0.258 | 41.01 | 0.903 |
| T (K) | Temkin Model | Dubinin–Radushkevich Model | |||||
| bT | KT | R2 | Qmax (mg/g) | E (KJ/mol) | R2 | ||
| 298 | 98.95 | 5.65 | 0.986 | 122.48 | 20.55 | 0.911 | |
| 308 | 106.83 | 5.37 | 0.989 | 116.56 | 20.47 | 0.892 | |
| 318 | 133.38 | 4.71 | 0.943 | 97.06 | 16.53 | 0.963 | |
| Adsorbents | BET (m2/g) | pH | Fitting Models | Qm (mg/g) | Ref. |
|---|---|---|---|---|---|
| M-ATP | 116.56 | 4 | Langmuir | 90.0 | [51] |
| Cys-d-FeOOH | 34 | 7 | Langmuir | 35.0 | [52] |
| MPTS-CNTs/Fe3O4 | 97 | 6 | Langmuir | 65.5 | [53] |
| Bi2O4/ZnO | – | 7 | Langmuir | 60.0 | [54] |
| M. pyrifera | – | 5 | Langmuir | 80.0 | [55] |
| o-benzenedithiol-modified cellulose | – | 6 | Langmuir | 86.0 | [56] |
| CoFe2O4@SiO2-EDTA | 20.09 | 7 | Langmuir | 103.3 | This work |
| C0 (mg/L) | ΔH0 (KJ/mol) | ΔS0 (J/mol/K) | ΔG0 (KJ/mol) | ||
|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | |||
| 20 | −13.83 | −26.61 | −5.814 | −5.691 | −5.270 |
| 30 | −15.89 | −37.42 | −4.678 | −4.495 | −3.921 |
| 40 | −19.98 | −33.75 | −3.826 | −3.787 | −3.138 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, K.; Guo, Y.; Shao, Q.; Zan, Q.; Bai, R. Removal of Mercury (II) by EDTA-Functionalized Magnetic CoFe2O4@SiO2 Nanomaterial with Core-Shell Structure. Nanomaterials 2019, 9, 1532. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9111532
Xia K, Guo Y, Shao Q, Zan Q, Bai R. Removal of Mercury (II) by EDTA-Functionalized Magnetic CoFe2O4@SiO2 Nanomaterial with Core-Shell Structure. Nanomaterials. 2019; 9(11):1532. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9111532
Chicago/Turabian StyleXia, Kai, Yongfu Guo, Qijun Shao, Qu Zan, and Renbi Bai. 2019. "Removal of Mercury (II) by EDTA-Functionalized Magnetic CoFe2O4@SiO2 Nanomaterial with Core-Shell Structure" Nanomaterials 9, no. 11: 1532. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9111532