Facile Fabrication of a Self-Healing Temperature-Sensitive Sensor Based on Ionogels and Its Application in Detection Human Breath
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. Characterization
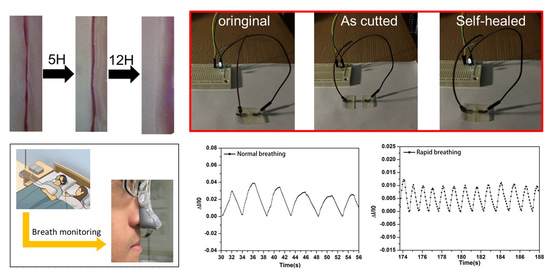
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Keplinger, C.; Suo, Z.; Sun, J.Y.; Foo, C.C.; Rothemund, P.; Whitesides, G.M. Stretchable, transparent, ionic conductors. Science 2013, 341, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Lee, S.W.; Yu, S.; Kim, H.; Chang, S.; Kang, D.; Hwang, I.; Kang, H.S.; Jeong, B.; Kim, E.H.; et al. Micropatterned Pyramidal Ionic Gels for Sensing Broad-Range Pressures with High Sensitivity. ACS Appl. Mater. Interfaces 2017, 9, 10128–10135. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Keplinger, C.; Whitesides, G.M.; Suo, Z. Ionic skin. Adv. Mater. 2015, 26, 7608–7614. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Wang, Q.; Sun, S.; Zhu, W.; Wu, P. A Bioinspired Mineral Hydrogel as a Self-Healable, Mechanically Adaptable Ionic Skin for Highly Sensitive Pressure Sensing. Adv. Mater. 2017, 29, 1700321. [Google Scholar] [CrossRef] [PubMed]
- Larson, C.; Peele, B.; Li, S.; Robinson, S.; Totaro, M.; Beccai, L.; Mazzolai, B.; Shepherd, R. Highly stretchable electroluminescent skin for optical signaling and tactile sensing. Science 2016, 351, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Jean, L.B.; Lydie, V.; André, V. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar]
- Yu, Q.; Wu, Y.; Li, D.; Cai, M.; Zhou, F.; Liu, W.J. Supramolecular ionogel lubricants with imidazolium-based ionic liquids bearing the urea group as gelator. Colloid Interface Sci. 2017, 487, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Carla, R.; Francesca, D.; Renato, N.; Zhang, M.; Weiss, R. Insights into the Formation and Structures of Molecular Gels by Diimidazolium Salt Gelators in Ionic Liquids or “Normal” Solvents. Chem.-Eur. J. 2016, 22, 11269–11282. [Google Scholar]
- Floriana, B.; Francesca, D.; Gunaratne, H.; Plechkova, N.; Seddon, K. “Sweet” ionic liquid gels: Materials for sweetening of fuels. Green Chem. 2018, 20, 4260. [Google Scholar]
- Carla, R.; Francesca, A.; Luka, D.; Nadka, T.; Renato, N.; Maurizio, P. Nitrogen-Doped Carbon Nanodots-Ionogels: Preparation, Characterization, and Radical Scavenging Activity. ACS Nano 2018, 12, 1296–1305. [Google Scholar]
- Carla, R.; Rossella, A.; Nadka, T.; Giuseppe, G.; Francesco, G.; Renato, N.; Alberto, S.; Paola, V.; Francesca, D. Supramolecular Hydro- and Ionogels: A Study of Their Properties and Antibacterial Activity. Chem.-Eur. J. 2017, 23, 16297–16311. [Google Scholar]
- Marullo, S.; Rizzo, C.; Dintcheva, N.; Giannici, F.; D’Anna, F.J. Ionic liquids gels: Soft materials for environmental remediation. Colloid Interface Sci. 2018, 517, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lu, J.J.; Yang, C.H.; Yang, J.H.; Zhou, J.; Chen, Y.M.; Suo, Z. Highly Stretchable and Transparent Ionogels as Nonvolatile Conductors for Dielectric Elastomer Transducers. ACS Appl. Mater. Interfaces 2014, 6, 7840–7845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, X.; Fu, W.; Xiao, L.; Ding, Y. Enhanced dc conductivity and conductivity relaxation in PVDF/ionic liquid composites. Mater. Lett. 2017, 206, 60–63. [Google Scholar]
- Susan, M.A.; Kaneko, T.; Noda, A.; Watanabe, M.J. Ion Gels Prepared by in Situ Radical Polymerization of Vinyl Monomers in an Ionic Liquid and Their Characterization as Polymer Electrolytes. J. Am. Chem. Soc. 2005, 127, 4976–4983. [Google Scholar] [CrossRef] [PubMed]
- Nie, B.; Li, R.; Cao, J.; Brandt, J.D.; Pan, T. Flexible Transparent Iontronic Film for Interfacial Capacitive Pressure Sensing. Adv. Mater. 2015, 27, 6055–6062. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Nguyen, G.T.M.; Plesse, C.; Vidal, F.; Jager, E.W.H. Highly Conductive, Photolithographically Patternable Ionogels for Flexible and Stretchable Electrochemical Devices. ACS Appl. Mater. Interfaces 2018, 10, 21601–21611. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, G.; Kim, D.; Ha, J.S. Air-Stable, High-Performance, Flexible Microsupercapacitor with Patterned Ionogel Electrolyte. ACS Appl. Mater. Interfaces 2015, 7, 4608–4615. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, N.; Zhang, B.; Zhang, B.; Song, J. Development of Self-Healing d-Gluconic Acetal-Based Supramolecular Ionogels for Potential Use as Smart Quasisolid Electrochemical Materials. ACS Appl. Mater. Interfaces 2018, 10, 5871–5879. [Google Scholar] [CrossRef] [PubMed]
- Tamate, R.; Hashimoto, K.; Horii, T.; Hirasawa, M.; Li, X.; Shibayama, M.; Watanabe, M. Self-Healing Micellar Ion Gels Based on Multiple Hydrogen Bonding. Adv. Mater. 2018, 30, 1802792. [Google Scholar] [CrossRef] [PubMed]
- Tsuyoshi, S.; Tomoyuki, Y.; Ute, Z.; Hagen, K.; Siegfried, B.; Ken, T.; Makoto, T.; Takayasu, S.; Takao, S. Organic Nonvolatile Memory Transistors for Flexible Sensor Arrays. Science 2009, 326, 1516–1519. [Google Scholar]
- Amjadi, M.; Pichitpajongkit, A.; Lee, S.; Ryu, S.; Park, I. Highly Stretchable and Sensitive Strain Sensor Based on Silver Nanowire–Elastomer Nanocomposite. ACS Nano 2014, 8, 5154–5163. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Liu, H.; Chen, Y.; Zheng, M.; Zhao, Y.; Kong, X.; Wang, Y.; Zhang, X.; Kong, X.; Wang, P.; et al. Highly Conductive, Air-Stable Silver Nanowire@Iongel Composite Films toward Flexible Transparent Electrodes. Adv. Mater. 2016, 28, 7167–7172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, F.; Peng, H.; Yan, J.; Pan, G. Flexible Highly Sensitive Pressure Sensor Based on Ionic Liquid Gel Film. ACS Omega 2018, 3, 3014–3021. [Google Scholar] [CrossRef]
- Liu, X.; Su, G.; Guo, Q.; Lu, C.; Tao, Z.; Zhou, C.; Zhang, X. Hierarchically Structured Self-Healing Sensors with Tunable Positive/Negative Piezoresistivity. Adv. Funct. Mater. 2018, 1706658. [Google Scholar] [CrossRef]
- Güder, F.; Ainla, A.; Redston, J.; Mosadegh, B.; Glavan, A.; Martin, T.J.; Whitesides, G.M. Paper-Based Electrical Respiration Sensor. Angew. Chem. 2016, 128, 5821–5826. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Zhu, R. Electronic Skin with Multifunction Sensors Based on Thermosensation. Adv. Mater. Technol. 2017, 1700183. [Google Scholar] [CrossRef]
- Jia, H.; Tao, X.; Wang, Y. Flexible and Self-Healing Thermoelectric Converters Based on Thermosensitive Liquids at Low Temperature Gradient. Adv. Electron. Mater. 2016, 1600136. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Zhang, S.; Zhang, Y.; Lin, Q.; Chen, Y.; Zhu, D.; Sun, L.; Chen, T. Facile Fabrication of a Self-Healing Temperature-Sensitive Sensor Based on Ionogels and Its Application in Detection Human Breath. Nanomaterials 2019, 9, 343. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9030343
Wang F, Zhang S, Zhang Y, Lin Q, Chen Y, Zhu D, Sun L, Chen T. Facile Fabrication of a Self-Healing Temperature-Sensitive Sensor Based on Ionogels and Its Application in Detection Human Breath. Nanomaterials. 2019; 9(3):343. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9030343
Chicago/Turabian StyleWang, Fengxia, Shaohui Zhang, Yunlin Zhang, Qihang Lin, Yun Chen, Dongfang Zhu, Lining Sun, and Tao Chen. 2019. "Facile Fabrication of a Self-Healing Temperature-Sensitive Sensor Based on Ionogels and Its Application in Detection Human Breath" Nanomaterials 9, no. 3: 343. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9030343