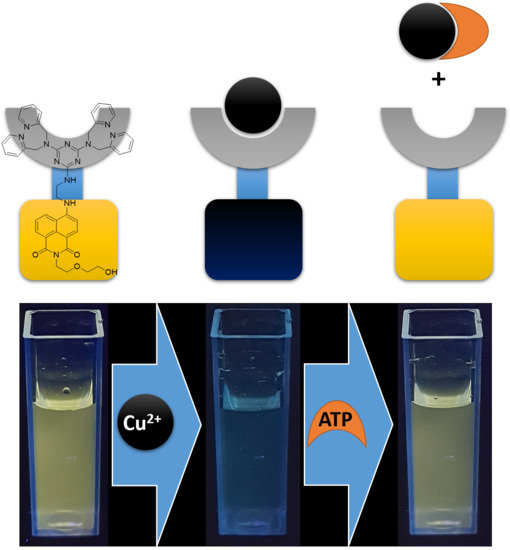
Selective Coordination of Cu2+ and Subsequent Anion Detection Based on a Naphthalimide-Triazine-(DPA)2 Chemosensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. UV-Vis and Fluorescence Titrations
2.3. Cell Viability and Fluorescence Microscopy Studies
3. Results and Discussion
3.1. Synthesis
3.2. UV-Vis and Fluorescence Studies
3.3. Anion Sensing Studies
3.4. Super-Resolution Structured Illumination Microscopy (SR-SIM) Live Cell Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Yoon, J. Recent progress on fluorescent chemosensors for metal ions. Inorg. Chim. Acta 2012, 381, 2–14. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busschaert, N.; Caltagirone, C.; Van Rossom, W.; Gale, P.A. Applications of supramolecular anion recognition. Chem. Rev. 2015, 115, 8038–8155. [Google Scholar] [CrossRef] [PubMed]
- Beer, P.D.; Gale, P.A. Anion recognition and sensing: The state of the art and future perspectives. Angew. Chem. Int. Ed. 2001, 40, 486–516. [Google Scholar] [CrossRef]
- Wrobel, A.T.; Johnstone, T.C.; Deliz Liang, A.; Lippard, S.J.; Rivera-Fuentes, P. A fast and selective near-infrared fluorescent sensor for multicolor imaging of biological nitroxyl (HNO). J. Am. Chem. Soc. 2014, 136, 4697–4705. [Google Scholar] [CrossRef]
- Pouessel, J.; Bazzicalupi, C.; Bencini, A.; Bernard, H.; Giorgi, C.; Handel, H.; Matera, I.; Le Bris, N.; Tripier, R.; Valtancoli, B. Exploring new molecular architectures for anion recognition: Synthesis and atp binding properties of new cyclam-based ditopic polyammonium receptors. Chem. An. Asian J. 2011, 6, 1582–1594. [Google Scholar] [CrossRef]
- Bhuyan, M.; Katayev, E.; Stadlbauer, S.; Nonaka, H.; Ojida, A.; Hamachi, I.; König, B. Rigid luminescent bis-zinc(ii)-bis-cyclen complexes for the detection of phosphate anions and non-covalent protein labeling in aqueous solution. European J. Org. Chem. 2011, 2807–2817. [Google Scholar] [CrossRef]
- Wang, M.Q.; Li, K.; Hou, J.T.; Wu, M.Y.; Huang, Z.; Yu, X.Q. BINOL-Based fluorescent sensor for recognition of cu(ii) and sulfide anion in water. J. Org. Chem. 2012, 77, 8350–8354. [Google Scholar] [CrossRef]
- Mizukami, S.; Nagano, T.; Urano, Y.; Odani, A.; Kikuchi, K. A Fluorescent anion sensor that works in neutral aqueous solution for bioanalytical application. J. Am. Chem. Soc. 2002, 124, 3920–3925. [Google Scholar] [CrossRef]
- García, V.; Fernández-Lodeiro, A.; Lamelas, R.; Macías, A.; Bastida, R.; Bértolo, E.; Núñez, C. Novel chromogenic macrocyclic molecular probes with logic gate function using anion/cation inputs. Dye. Pigment. 2014, 110, 143–151. [Google Scholar] [CrossRef]
- Wong, J.K.H.; Todd, M.H.; Rutledge, P.J. Recent advances in macrocyclic fluorescent probes for ion sensing. Molecules 2017, 22, 200. [Google Scholar] [CrossRef] [Green Version]
- Mesquita, L.M.; André, V.; Esteves, C.V.; Palmeira, T.; Berberan-Santos, M.N.; Mateus, P.; Delgado, R. Dinuclear Zinc(II) macrocyclic complex as receptor for selective fluorescence sensing of pyrophosphate. Inorg. Chem. 2016, 55, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.; Núñez-Montenegro, A.; Mateus, P.; Delgado, R. Monitoring inorganic pyrophosphatase activity with the fluorescent dizinc(ii) complex of a macrocycle bearing one dansylamidoethyl antenna. Dalton Trans. 2020, 49, 9487–9494. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Ojida, A.; Hamachi, I. Molecular recognition, fluorescence sensing, and biological assay of phosphate anion derivatives using artificial Zn(II)-Dpa complexes. Chem. Commun. 2009, 141–152. [Google Scholar] [CrossRef]
- Ojida, A.; Mito-Oka, Y.; Sada, K.; Hamachi, I. Molecular recognition and fluorescence sensing of monophosphorylated peptides in aqueous solution by bis(zinc(ii)-dipicolylamine)-based artificial receptors. J. Am. Chem. Soc. 2004, 126, 2454–2463. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.T.; Liu, X.; Jolliffe, K.A. Anion recognition and sensing with Zn(ii)-dipicolylamine complexes. Chem. Soc. Rev. 2012, 41, 4928–4965. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Ou, D.; Li, Q.; Li, Z. An indirect approach for anion detection: The displacement strategy and its application. Chem. Commun. 2012, 48, 8462–8477. [Google Scholar] [CrossRef] [PubMed]
- Moro, A.J.; Cywinski, P.J.; Körsten, S.; Mohr, G.J. An ATP fluorescent chemosensor based on a Zn(ii)-complexed dipicolylamine receptor coupled with a naphthalimide chromophore. Chem. Commun. 2010, 46, 1085–1087. [Google Scholar] [CrossRef]
- Moro, A.J.; Schmidt, J.; Doussineau, T.; Lapresta-Fernandéz, A.; Wegener, J.; Mohr, G.J. Surface-functionalized fluorescent silica nanoparticles for the detection of ATP. Chem. Commun. 2011, 47, 6066–6068. [Google Scholar] [CrossRef] [Green Version]
- Trupp, S.; Schweitzer, A.; Mohr, G.J. A fluorescent water-soluble naphthalimide-based receptor for saccharides with highest sensitivity in the physiological pH range. Org. Biomol. Chem. 2006, 4, 2965–2968. [Google Scholar] [CrossRef] [PubMed]
- Olmsted, J. Calorimetric determinations of absolute fluorescence quantum yields. J. Phys. Chem. 1979, 83, 2581–2584. [Google Scholar] [CrossRef]
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.M.; Almada, P.; Henriques, R. High-content 3D multicolor super-resolution localization microscopy. Methods Cell Biol. 2015, 125, 95–117. [Google Scholar] [PubMed]
- Qi, X.; Kim, S.K.; Han, S.J.; Xu, L.; Jee, A.Y.; Kim, H.N.; Lee, C.; Kim, Y.; Lee, M.; Kim, S.J.; et al. New BODIPY-triazine based tripod fluorescent systems. Tetrahedron Lett. 2008, 49, 261–264. [Google Scholar] [CrossRef]
- Weng, Y.Q.; Yue, F.; Zhong, Y.R.; Ye, B.H. A copper(II) ion-selective on-off-type fluoroionophore based on zinc porphyrin-dipyridylamino. Inorg. Chem. 2007, 46, 7749–7755. [Google Scholar] [CrossRef]
- Xie, J.; Ménand, M.; Maisonneuve, S.; Métivier, R. Synthesis of bispyrenyl sugar-aza-crown ethers as new fluorescent molecular sensors for Cu(II). J. Org. Chem. 2007, 72, 5980–5985. [Google Scholar] [CrossRef]
- Sivaraman, G.; Iniya, M.; Anand, T.; Kotla, N.G.; Sunnapu, O.; Singaravadivel, S.; Gulyani, A.; Chellappa, D. Chemically diverse small molecule fluorescent chemosensors for copper ion. Coord. Chem. Rev. 2018, 357, 50–104. [Google Scholar] [CrossRef]
- Anderegg, G.; Hubmann, E.; Podder, N.G.; Wenk, F. Pyridinderivate als komplexbildner. xi. die thermodynamik der metallkomplexbildung mit bis-, tris- und tetrakis[(2-pyridyl)methyl]-aminen. Helv. Chim. Acta 1977, 60, 123–140. [Google Scholar] [CrossRef]
- Smith, R.M.; Martell, A.E.; Motekaitis, R.J. NIST Critically Selected Stability Constants of Metal Complexes Database. NIST Stand. Ref. Database 46 2004; National Institute of Standard and Technology: Gaithersburg, MD, USA, 2004. [Google Scholar]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moro, A.J.; Santos, M.; Outis, M.; Mateus, P.; Pereira, P.M. Selective Coordination of Cu2+ and Subsequent Anion Detection Based on a Naphthalimide-Triazine-(DPA)2 Chemosensor. Biosensors 2020, 10, 129. https://0-doi-org.brum.beds.ac.uk/10.3390/bios10090129
Moro AJ, Santos M, Outis M, Mateus P, Pereira PM. Selective Coordination of Cu2+ and Subsequent Anion Detection Based on a Naphthalimide-Triazine-(DPA)2 Chemosensor. Biosensors. 2020; 10(9):129. https://0-doi-org.brum.beds.ac.uk/10.3390/bios10090129
Chicago/Turabian StyleMoro, Artur J., Miguel Santos, Mani Outis, Pedro Mateus, and Pedro M. Pereira. 2020. "Selective Coordination of Cu2+ and Subsequent Anion Detection Based on a Naphthalimide-Triazine-(DPA)2 Chemosensor" Biosensors 10, no. 9: 129. https://0-doi-org.brum.beds.ac.uk/10.3390/bios10090129