Voltammetric Determination of 3-Methylmorphine Using Glassy Carbon Electrode Modified with rGO and Bismuth Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Preparation of rGO and Modification of GCE with rGO and Bismuth Film
2.3. Preparation of Synthetic Urine, Bovine Serum and River Water Samples
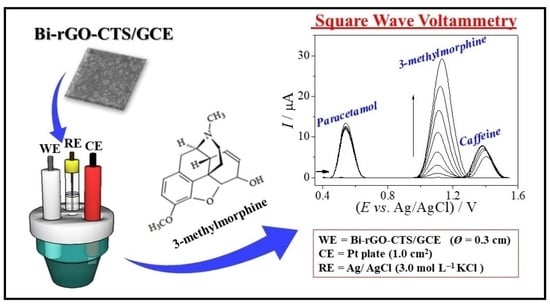
2.4. Analytical Procedure
3. Results and Discussion
3.1. Material Characterization
3.2. Analytical Response
3.3. Calibration Curve
3.4. Study of Repeatability and the Influence of Possible Interferents
3.5. Analysis of 3-Methylmorphine in Synthetic Urine, Bovine Serum and River Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verrinder, E.; Wester, N.; Leppänen, E.; Lilius, T.; Kalso, E.; Mikladal, B.r.; Varjos, I.; Koskinen, J.; Laurila, T. Electrochemical Detection of Morphine in Untreated Human Capillary Whole Blood. ACS Omega 2021, 6, 11563–11569. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, R.; Noviana, E.; Fauziati, R.; Carrão, D.B.; Gandhi, F.A.; Majid, M.A.; Saputri, F.A. A Review of Analytical Methods for Codeine Determination. Molecules 2021, 26, 800. [Google Scholar] [CrossRef] [PubMed]
- Khairy, M.; Mahmoud, B.G.; Banks, C.E. Simultaneous determination of codeine and its co-formulated drugs acetaminophen and caffeine by utilising cerium oxide nanoparticles modified screen-printed electrodes. Sens. Actuators B Chem. 2018, 259, 142–154. [Google Scholar] [CrossRef]
- Mohseni, N.; Bahram, M. Mean centering of ratio spectra for colorimetric determination of morphine and codeine in pharmaceuticals and biological samples using melamine modified gold nanoparticles. Anal. Methods 2016, 8, 6739–6747. [Google Scholar] [CrossRef]
- Lodha, A.; Pandya, A.; Sutariya, P.G.; Menon, S.K. A smart and rapid colorimetric method for the detection of codeine sulphate, using unmodified gold nanoprobe. RSC Adv. 2014, 4, 50443–50448. [Google Scholar] [CrossRef]
- Saeed, A.; Ahmed, N.Q. Simultaneous spectrophotometric determination of paracetamol and codeine. Eur. J. Pharm. Med. Res. 2018, 5, 11–15. [Google Scholar]
- Lotfi, A.; Karimi, S.; Hassanzadeh, J. Preconcentration of codeine in pharmaceutical and human urine samples by multi-walled carbon nanotubes and its spectrophotometric determination. Can. J. Chem. 2016, 94, 857–864. [Google Scholar] [CrossRef]
- Grabenauer, M.; Moore, K.N.; Bynum, N.D.; White, R.M.; Mitchell, J.M.; Hayes, E.D.; Flegel, R. Development of a Quantitative LC–MS-MS Assay for Codeine, Morphine, 6-Acetylmorphine, Hydrocodone, Hydromorphone, Oxycodone and Oxymorphone in Neat Oral Fluid. J. Anal. Toxicol. 2018, 42, 392–399. [Google Scholar] [CrossRef]
- Plotnikov, A.N.; Karpenko, Y.N.; Vikhareva, E.V.; Tyumina, E.A.; Richkova, M.I.; Selyaninov, A.A. Determination of Codeine Phosphate in the Culture Fluid of Rhodococcus by High-Performance Liquid Chromatography. Mosc. Univ. Chem. Bull. 2017, 72, 328–332. [Google Scholar] [CrossRef]
- Abdul Keyon, A.S.; Miskam, M.; Ishak, N.S.; Mahat, N.A.; Mohamed Huri, M.A.; Abdul Wahab, R.; Chandren, S.; Abdul Razak, F.I.; Ng, N.-T.; Ali, T.G. Capillary electrophoresis for the analysis of antidepressant drugs: A review. J. Sep. Sci. 2019, 42, 906–924. [Google Scholar] [CrossRef]
- Baciu, T.; Botello, I.; Borrull, F.; Calull, M.; Aguilar, C. Capillary electrophoresis and related techniques in the determination of drugs of abuse and their metabolites. TrAC Trends Anal. Chem. 2015, 74, 89–108. [Google Scholar] [CrossRef]
- Santos, A.M.; Silva, T.A.; Vicentini, F.C.; Fatibello-Filho, O. Flow injection analysis system with electrochemical detection for the simultaneous determination of nanomolar levels of acetaminophen and codeine. Arab. J. Chem. 2020, 13, 335–345. [Google Scholar] [CrossRef]
- Wong, A.; Riojas, A.C.; Baena-Moncada, A.M.; Sotomayor, M.D.P.T. A new electrochemical platform based on carbon black paste electrode modified with α-cyclodextrin and hierarchical porous carbon used for the simultaneous determination of dipyrone and codeine. Microchem. J. 2021, 164, 106032. [Google Scholar] [CrossRef]
- Khairy, M. A synergetic effect of cerium oxide nanocubes and gold nanoparticles for developing a new photoelectrochemical sensor of codeine drug. J. Electroanal. Chem. 2021, 895, 115517. [Google Scholar] [CrossRef]
- Wong, A.; Santos, A.M.; Fatibello-Filho, O. Determination of piroxicam and nimesulide using an electrochemical sensor based on reduced graphene oxide and PEDOT:PSS. J. Electroanal. Chem. 2017, 799, 547–555. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene Based Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- De Figueiredo-Filho, L.C.S.; dos Santos, V.B.; Janegitz, B.C.; Guerreiro, T.B.; Fatibello-Filho, O.; Faria, R.C.; Marcolino-Junior, L.H. Differential Pulse Voltammetric Determination of Paraquat Using a Bismuth-Film Electrode. Electroanalysis 2010, 22, 1260–1266. [Google Scholar] [CrossRef]
- Lau, I.-W.; Mok, C.-S. High-performance liquid chromatographic determination of active ingredients in cough-cold syrups with indirect conductometric detection. J. Chromatogr. A 1995, 693, 45–54. [Google Scholar] [CrossRef]
- Wong, A.; Santos, A.M.; Silva, T.A.; Fatibello-Filho, O. Simultaneous determination of isoproterenol, acetaminophen, folic acid, propranolol and caffeine using a sensor platform based on carbon black, graphene oxide, copper nanoparticles and PEDOT:PSS. Talanta 2018, 183, 329–338. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Zheng, Q.-B.; Qiu, H.-X.; Li, J.; Yang, J.-H. A simple method for the reduction of graphene oxide by sodium borohydride with CaCl2 as a catalyst. New Carbon Mater. 2015, 30, 41–47. [Google Scholar] [CrossRef]
- Rowley-Neale, S.J.; Randviir, E.P.; Abo Dena, A.S.; Banks, C.E. An overview of recent applications of reduced graphene oxide as a basis of electroanalytical sensing platforms. Appl. Mater. Today 2018, 10, 218–226. [Google Scholar] [CrossRef]
- Laube, N.; Mohr, B.; Hesse, A. Laser-probe-based investigation of the evolution of particle size distributions of calcium oxalate particles formed in artificial urines. J. Cryst. Growth 2001, 233, 367–374. [Google Scholar] [CrossRef]
- Bao-Kang, J.; Jian-Rong, Z.; Zu-Xun, Z. Theory and application of cyclic voltammetry for measurement of fast electrode kinetics at microdisk electrode. Chin. J. Chem. 1996, 14, 338–347. [Google Scholar] [CrossRef]
- Lavagnini, I.; Antiochia, R.; Magno, F. An Extended Method for the Practical Evaluation of the Standard Rate Constant from Cyclic Voltammetric Data. Electroanalysis 2004, 16, 505–506. [Google Scholar] [CrossRef]
- Fatahi, A.; Malakooti, R.; Shahlaei, M. Electrocatalytic oxidation and determination of dexamethasone at an Fe3O4/PANI–CuII microsphere modified carbon ionic liquid electrode. RSC Adv. 2017, 7, 11322–11330. [Google Scholar] [CrossRef] [Green Version]
- Garrido, J.M.P.J.; Delerue-Matos, C.; Borges, F.; Macedo, T.R.A.; Oliveira-Brett, A.M. Voltammetric Oxidation of Drugs of Abuse II. Codeine and Metabolites. Electroanalysis 2004, 16, 1427–1433. [Google Scholar] [CrossRef] [Green Version]
- Asturias-Arribas, L.; Asunción Alonso-Lomillo, M.; Domínguez-Renedo, O.; Julia Arcos-Martínez, M. Screen-printed biosensor based on the inhibition of the acetylcholinesterase activity for the determination of codeine. Talanta 2013, 111, 8–12. [Google Scholar] [CrossRef]
- Bagheri, H.; Khoshsafar, H.; Afkhami, A.; Amidi, S. Sensitive and simple simultaneous determination of morphine and codeine using a Zn2SnO4 nanoparticle/graphene composite modified electrochemical sensor. New J. Chem. 2016, 40, 7102–7112. [Google Scholar] [CrossRef]
- Simioni, N.B.; Oliveira, G.G.; Vicentini, F.C.; Lanza, M.R.V.; Janegitz, B.C.; Fatibello-Filho, O. Nanodiamonds stabilized in dihexadecyl phosphate film for electrochemical study and quantification of codeine in biological and pharmaceutical samples. Diam. Relat. Mater. 2017, 74, 191–196. [Google Scholar] [CrossRef]
- Švorc, Ľ.; Sochr, J.; Svítková, J.; Rievaj, M.; Bustin, D. Rapid and sensitive electrochemical determination of codeine in pharmaceutical formulations and human urine using a boron-doped diamond film electrode. Electrochim. Acta 2013, 87, 503–510. [Google Scholar] [CrossRef]
- Garazhian, E.; Shishehbore, M.R. A New Sensitive Sensor for Simultaneous Differential Pulse Voltammetric Determination of Codeine and Acetaminophen Using a Hydroquinone Derivative and Multiwall Carbon Nanotubes Carbon Paste Electrode. Int. J. Anal. Chem. 2015, 2015, 783157. [Google Scholar] [CrossRef] [PubMed]
- Asturias-Arribas, L.; Asunción Alonso-Lomillo, M.; Domínguez-Renedo, O.; Julia Arcos-Martínez, M. Cytochrome P450 2D6 based electrochemical sensor for the determination of codeine. Talanta 2014, 129, 315–319. [Google Scholar] [CrossRef] [PubMed]









| Electrode | Method | Linear Range (µmol L−1) | LOD (µmol L−1) | Sensitivity (µA mol−1 L) | Presence | Ref. |
|---|---|---|---|---|---|---|
| CD-HPC/CBPE 1 | SWV | 0.5–38 | 0.0095 | 0.047 | Dipyrone | [13] |
| AChE-TTF-SPCEs 2 | Chrono-amperometry | 20–200 | 0.02 | No | - | [27] |
| Zn2SnO4—GO/CPE 3 | DPV | 0.02–15 | 0.009 | 2.938 | Morphine | [28] |
| ND-DHP/GCE 4 | SWV | 0.299–10.8 | 0.0545 | 0.0745 | - | [29] |
| BDD 5 | DPV | 0.1–60 | 0.08 | 0.155 | - | [30] |
| HTP-MWCNT/CPE 6 | DPV | 0.2–34.1 34.1–844.7 | 0.063 | 0.0288 0.009 | Acetaminophen | [31] |
| CYP2D6-SPCEs 7 | Chrono-amperometry | 5.0–35.0 | 4.9 | - | - | [32] |
| Bi-rGO-CTS/GCE | SWV | 0.25–8.2 | 0.02 | 3.5 | Paracetamol and caffeine | This work |
| Samples | [3-Methylmorphine]/mol L−1 | Recovery ** (Sensor, %) | ||
|---|---|---|---|---|
| Added | Found * | Comparative Method * | ||
| Synthetic urine | 1.0 × 10−7 | (1.02 ± 0.08) × 10−7 | (1.05 ± 0.05) × 10−7 | 102 |
| 1.0 × 10−6 | (0.99 ± 0.06) × 10−6 | (0.95 ± 0.04) × 10−7 | 99.0 | |
| Serum | 1.0 × 10−7 | (1.03 ± 0.09) × 10−7 | (1.02 ± 0.04) × 10−6 | 103 |
| 1.0 × 10−6 | (1.05 ± 0.07) × 10−6 | (1.03 ± 0.06) × 10−6 | 105 | |
| River water | 1.0 × 10−7 | (1.00 ± 0.09) × 10−7 | (1.00 ± 0.03) × 10−7 | 100 |
| 1.0 × 10−6 | (0.95 ± 0.06) × 10−6 | (0.98 ± 0.05) × 10−6 | 95.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, A.; Santos, A.M.; Proença, C.A.; Baldo, T.A.; Feitosa, M.H.A.; Moraes, F.C.; Sotomayor, M.D.P.T. Voltammetric Determination of 3-Methylmorphine Using Glassy Carbon Electrode Modified with rGO and Bismuth Film. Biosensors 2022, 12, 860. https://0-doi-org.brum.beds.ac.uk/10.3390/bios12100860
Wong A, Santos AM, Proença CA, Baldo TA, Feitosa MHA, Moraes FC, Sotomayor MDPT. Voltammetric Determination of 3-Methylmorphine Using Glassy Carbon Electrode Modified with rGO and Bismuth Film. Biosensors. 2022; 12(10):860. https://0-doi-org.brum.beds.ac.uk/10.3390/bios12100860
Chicago/Turabian StyleWong, Ademar, Anderson M. Santos, Camila A. Proença, Thaísa A. Baldo, Maria H. A. Feitosa, Fernando C. Moraes, and Maria D. P. T. Sotomayor. 2022. "Voltammetric Determination of 3-Methylmorphine Using Glassy Carbon Electrode Modified with rGO and Bismuth Film" Biosensors 12, no. 10: 860. https://0-doi-org.brum.beds.ac.uk/10.3390/bios12100860