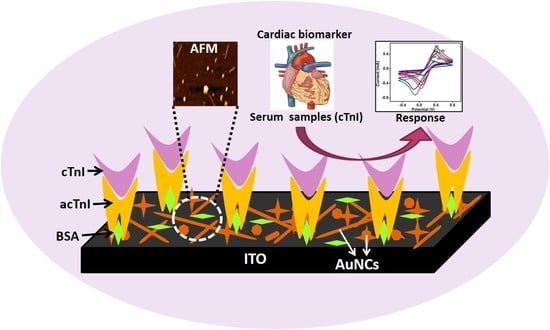
Template Free Anisotropically Grown Gold Nanocluster Based Electrochemical Immunosensor for Ultralow Detection of Cardiac Troponin I
Abstract
:1. Introduction
- (i)
- 0–0.4 ng/mL: healthy person
- (ii)
- Between 0.4 and 4 ng mL−1: minor heart disorder and unstable angina
- (iii)
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Gold Seeds
2.3. Preparation of APTMS/ITO Electrodes
2.4. Fabrication of AuNCs (Gold Nanoclusters) Modified ITO Electrode (AuNCs/APTMS/ITO)
2.5. Development of BSA/acTnI/Cys/AuNCs/APTMS/ITO Immunoelectrode
2.6. Optimization of Operational Parameters of BSA/acTnI/Cys/AuNCs/APTMS/ITO Immuno-Electrodes
2.7. Detection of cTnI Using Developed BSA/acTnI/Cys/AuNCs/APTMS/ITO Immunoelectrode
2.8. Determination of cTnI Using Developed BSA/acTnI/Cys/AuNCs/APTMS/ITO Immunoelectrodes in Spiked Serum Sample
2.9. Stability Studies
3. Characterization
4. Result and Discussion
4.1. Structural and Morphological Analysis
4.1.1. SEM and AFM Analysis
4.1.2. UV-Visible Spectroscopy
4.1.3. IR Spectroscopy
4.2. Electrochemical Characterization of the Developed Electrodes
4.2.1. Cyclic Voltammetry Study
4.2.2. Optimization of Variables
4.2.3. Immunosensor—Response Studies
4.2.4. Stability and Interference Study of Immunoelectrode
4.2.5. Measurement of cTnI in Spiked Serum Samples
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elechiguerra, J.L.; Reyes-Gasga, J.; Yacaman, M.J. The Role of Twinning in Shape Evolution of Anisotropic Noble Metal Nanostructures. J. Mater. Chem. 2006, 16, 3906–3919. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Y.; Xue, X.; Huo, S.; Chen, F.; Zou, G.; Liang, X.-J. Seedless Synthesis of High Aspect Ratio Gold Nanorods with High Yield. J. Mater. Chem. A 2014, 2, 3528. [Google Scholar] [CrossRef]
- Alagiri, M.; Rameshkumar, P.; Pandikumar, A. Gold Nanorod-Based Electrochemical Sensing of Small Biomolecules: A Review. Microchim. Acta 2017, 184, 3069–3092. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, W.; Foroushani, A.D.; Wang, H.; Li, D.; Liu, J.; Barrow, C.J.; Wang, X.; Yang, W. New Gold Nanostructures for Sensor Applications: A Review. Materials 2014, 7, 5169–5201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, B.B.; Singh, R.; Kumar, A. Gold Nanorods vs. Gold Nanoparticles: Application in Electrochemical Sensing of Cytosine β-D-Arabinoside Using Metal Ion Mediated Molecularly Imprinted Polymer. RSC Adv. 2016, 6, 80679–80691. [Google Scholar] [CrossRef]
- Manfroi, W.C.; Peukert, C.; Berti, C.B.; Noer, C.; Gutierres, D.D.A.; Silva, F.T.B.G.C. Da Acute Myocardial Infarction: The First Manifestation of Ischemic Heart Disease and Relation to Risk Factors. Arq. Bras. Cardiol. 2002, 78, 392–395. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, D.; Kaur, I.; Kumar, A. Ultrasensitive Cardiac Troponin I Antibody Based Nanohybrid Sensor for Rapid Detection of Human Heart Attack. Int. J. Biol. Macromol. 2017, 95, 505–510. [Google Scholar] [CrossRef]
- Liu, Q.; Aroonyadet, N.; Song, Y.; Wang, X.; Cao, X.; Liu, Y.; Cong, S.; Wu, F.; Thompson, M.E.; Zhou, C. Highly Sensitive and Quick Detection of Acute Myocardial Infarction Biomarkers Using In2O3 Nanoribbon Biosensors Fabricated Using Shadow Masks. ACS Nano 2016, 10, 10117–10125. [Google Scholar] [CrossRef]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Heart Disease and Stroke Statistics-2014 Update: A Report from the American Heart Association. Circulation 2014, 129, e28–e292. [Google Scholar] [CrossRef] [Green Version]
- Wilkins, E.; Wilson, L.; Wickramasinghe, K.; Bhatnagar, P.; Leal, J.; Luengo-Fernandez, R.; Burns, R.; Rayner, M.; Townsend, N. European Cardiovascular Disease Statistics 2017, European Heart Network, Brussels. Eur. Cardiovasc. Dis. Stat. 2017, 34, 3028–3034. [Google Scholar]
- Babuin, L.; Jaffe, A.S. Troponin: The Biomarker of Choice for the Detection of Cardiac Injury. CMAJ 2005, 173, 1191–1202. [Google Scholar] [CrossRef]
- Fonseca, R.A.S.; Ramos-Jesus, J.; Kubota, L.T.; Dutra, R.F. A Nanostructured Piezoelectric Immunosensor for Detection of Human Cardiac Troponin T. Sensors 2011, 11, 10785–10797. [Google Scholar] [CrossRef] [Green Version]
- Zapp, E.; Westphal, E.; Gallardo, H.; de Souza, B.; Vieira, I.C. Liquid Crystal and Gold Nanoparticles Applied to Electrochemical Immunosensor for Cardiac Biomarker. Biosens. Bioelectron. 2014, 59, 127–133. [Google Scholar] [CrossRef]
- Abdolrahim, M.; Rabiee, M.; Alhosseini, S.N.; Tahriri, M.; Yazdanpanah, S.; Tayebi, L. Development of Optical Biosensor Technologies for Cardiac Troponin Recognition. Anal. Biochem. 2015, 485, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Basu, T. Gold Nanopellets a Unique Platform for Electrochemical Ultra-Low Detection of Ochratoxin A. Food Sci. Nutr. 2018, 4, 32. [Google Scholar] [CrossRef]
- Ferrieres, G.; Calzolari, C.; Mani, J.C.; Laune, D.; Trinquier, S.; Laprade, M.; Larue, C.; Pau, B.; Granier, C. Human Cardiac Troponin I: Precise Identification of Antigenic Epitopes and Prediction of Secondary Structure. Clin. Chem. 1998, 44, 487–493. [Google Scholar] [CrossRef]
- Antman, E.M.; Tanasijevic, M.J.; Thompson, B.; Schactman, M.; McCabe, C.H.; Cannon, C.P.; Fischer, G.; Fung, Y.; Thompson, C.; Wybenga, D.; et al. Cardiac-Specific Troponin I Levels to Predict the Risk of Mortality in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 1996, 335, 1342–1349. [Google Scholar] [CrossRef] [Green Version]
- Bonyár, A. Label-Free Nucleic Acid Biosensing Using Nanomaterial-Based Localized Surface Plasmon Resonance Imaging: A Review. ACS Appl. Nano Mater. 2020, 3, 8506–8521. [Google Scholar] [CrossRef]
- Mustafa, F.; Andreescu, S. Nanotechnology-Based Approaches for Food Sensing and Packaging Applications. RSC Adv. 2020, 10, 19309–19336. [Google Scholar] [CrossRef]
- Mokhtarzadeh, A.; Eivazzadeh-Keihan, R.; Pashazadeh, P.; Hejazi, M.; Gharaatifar, N.; Hasanzadeh, M.; Baradaran, B.; de la Guardia, M. Nanomaterial-Based Biosensors for Detection of Pathogenic Virus. TrAC Trends Anal. Chem. 2017, 97, 445–457. [Google Scholar] [CrossRef]
- Nezami, A.; Dehghani, S.; Nosrati, R.; Eskandari, N.; Taghdisi, S.M.; Karimi, G. Nanomaterial-Based Biosensors and Immunosensors for Quantitative Determination of Cardiac Troponins. J. Pharm. Biomed. Anal. 2018, 159, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Abdorahim, M.; Rabiee, M.; Alhosseini, S.N.; Tahriri, M.; Yazdanpanah, S.; Alavi, S.H.; Tayebi, L. Nanomaterials-Based Electrochemical Immunosensors for Cardiac Troponin Recognition: An Illustrated Review. TrAC Trends Anal. Chem. 2016, 82, 337–347. [Google Scholar] [CrossRef]
- Li, Z.; Ma, K.; Cheng, Z.; Yan, C.; Liu, G. Fabrication of Electrochemical Immunosensor for Cardiac Biomarker Troponin I Determination and Its Potential for Acute Myocardial Infarction Diagnosis. Int. J. Electrochem. Sci. 2017, 12, 2389–2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatnagar, D.; Kumar, V.; Kumar, A.; Kaur, I. Graphene Quantum Dots FRET Based Sensor for Early Detection of Heart Attack in Human. Biosens. Bioelectron. 2016, 79, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Arunadevi, N.; Devipriya, S.; Vairam, S. The Acid Adducts Hydrazinium 2-Hydroxybenzoate-2-Hydroxybenzoic Acid (1/1) and Hydrazinium 3-Hydroxy-2-Naphthoate-3-Hydroxy-2-Naphthoic Acid (1/1). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2012, 68, o61–o64. [Google Scholar] [CrossRef]
- Wei, Z.; Mieszawska, A.J.; Zamborini, F.P. Synthesis and Manipulation of High Aspect Ratio Gold Nanorods Grown Directly on Surfaces. Langmuir 2004, 20, 4322–4326. [Google Scholar] [CrossRef]
- Arunadevi, N.; Kanchana, P.; Hemapriya, V.; Sankaran, S.S.; Mayilsamy, M.; Balakrishnan, P.D.; Chung, I.-M.; Mayakrishnan, P. Synthesis and Crystal Growth of Cadmium Naphthoate Crystal for Second Order Non-Linear Optics and Cytotoxic Activity. J. Dispers. Sci. Technol. 2021, 43, 2192–2208. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, J.; Agrawal, V.V.; Ahamad, M.; Malhotra, B.D. Biocompatible Self-Assembled Monolayer Platform Based on (3-Glycidoxypropyl)Trimethoxysilane for Total Cholesterol Estimation. Anal. Methods 2011, 3, 2237–2245. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, R.; Singh, J.; Solanki, P.R.; Manaka, T.; Iwamoto, M.; Basu, T.; Malhotra, B.D. Label-Free Piezoelectric Immunosensor Decorated with Gold Nanoparticles: Kinetic Analysis and Biosensing Application. Sens. Actuators B Chem. 2016, 222, 804–814. [Google Scholar] [CrossRef]
- Arunadevi, N.; Swathika, M.; Devi, B.P.; Kanchana, P.; Sundari, S.S.; Kirubavathy, S.J.; Subhapriya, P.; Kumar, E.R. Structural, Optical, Thermal, Biological and Molecular Docking Studies of Guanidine Based Naphthoate Metal Complexes. Surf. Interfaces 2021, 24, 101094. [Google Scholar] [CrossRef]
- Singh, J.; Roychoudhury, A.; Srivastava, M.; Solanki, P.R.; Lee, D.W.; Lee, S.H.; Malhotra, B.D. A Highly Efficient Rare Earth Metal Oxide Nanorods Based Platform for Aflatoxin Detection. J. Mater. Chem. B 2013, 1, 4493–4503. [Google Scholar] [CrossRef]
- Bhalla, V.; Carrara, S.; Sharma, P.; Nangia, Y.; Raman Suri, C. Gold Nanoparticles Mediated Label-Free Capacitance Detection of Cardiac Troponin I. Sens. Actuators B Chem. 2012, 161, 761–768. [Google Scholar] [CrossRef]
- Singh, N.; Rai, P.; Ali, M.A.; Kumar, R.; Sharma, A.; Malhotra, B.D.; John, R. A Hollow-Nanosphere-Based Microfluidic Biosensor for Biomonitoring of Cardiac Troponin I. J. Mater. Chem. B 2019, 7, 3826–3839. [Google Scholar] [CrossRef]
- Regan, B.; O’Kennedy, R.; Collins, D. Point-of-Care Compatibility of Ultra-Sensitive Detection Techniques for the Cardiac Biomarker Troponin I—Challenges and Potential Value. Biosensors 2018, 8, 114. [Google Scholar] [CrossRef] [Green Version]
- Periyakaruppan, A.; Gandhiraman, R.P.; Meyyappan, M.; Koehne, J.E. Label-Free Detection of Cardiac Troponin-I Using Carbon Nanofiber Based Nanoelectrode Arrays. Anal. Chem. 2013, 85, 3858–3863. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M. Antibody Storage and Antibody Shelf Life. Mater. Methods 2012, 2, 120. [Google Scholar] [CrossRef]







| S.No. | Matrix | Detection Method | Detection Range | LOD | Sensitivity | Ref. |
|---|---|---|---|---|---|---|
| 1 | cTnI antibody conjugated nanohybrid modified gold electrode | Electrochemical | 10−6–10 ng/mL | 20 fg/mL | 109.23 μA cm−2 μg−1 | [31] |
| 2 | acTnI/PDDA/RGO nanocomposite | Electrochemical | 0.1–10 ng/mL | 0.024 ng/mL | [21] | |
| 3 | acTnI/(2-ABA) functionalized graphene | Electrochemical | 0.01–1 ng/mL | 0.01 ng/mL | [32] | |
| 4 | acTnI/GNPs Matrix/SPE | Electrochemical | 0.2–12.5 ng/mL | 0.2 ng/mL | [25] | |
| 5 | acTnI/GNP/ITO | Electrochemical | 1 to 100 ng/mL | 1 ng/mL | [33] | |
| 6 | anti-cTnI/afGQDs | FRET | 0.001 to 1000 ng/mL | 0.192 pg/mL | [22] | |
| 7 | anti-cTnI/CNFs | Electrochemical | 0.25–100 ng/mL | 0.2 ng/mL | [34] | |
| 8 | acTnI/AuNCs/ITO | Electrochemical | 0.06–100 ng/mL | 0.043 ng/mL | 2.2 × 10−4 A ng−1 mL cm−2 | Present work |
| Sample no. | Actual Spiked Concentration (ng/mL) | Experimental Concentration (ng/mL) | % Error | Relative Standard Deviation |
|---|---|---|---|---|
| 1 | 70 | 52 | 25% | 6.9% |
| 2 | 0.8 | 0.85 | 6.25% | 5.16% |
| 3 | 0.5 | 0.43 | 14% | 5.34% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisar, S.; Chansi; Mathur, A.; Basu, T.; Singh, K.R.; Singh, J. Template Free Anisotropically Grown Gold Nanocluster Based Electrochemical Immunosensor for Ultralow Detection of Cardiac Troponin I. Biosensors 2022, 12, 1144. https://0-doi-org.brum.beds.ac.uk/10.3390/bios12121144
Nisar S, Chansi, Mathur A, Basu T, Singh KR, Singh J. Template Free Anisotropically Grown Gold Nanocluster Based Electrochemical Immunosensor for Ultralow Detection of Cardiac Troponin I. Biosensors. 2022; 12(12):1144. https://0-doi-org.brum.beds.ac.uk/10.3390/bios12121144
Chicago/Turabian StyleNisar, Sumaya, Chansi, Ashish Mathur, Tinku Basu, Kshitij RB Singh, and Jay Singh. 2022. "Template Free Anisotropically Grown Gold Nanocluster Based Electrochemical Immunosensor for Ultralow Detection of Cardiac Troponin I" Biosensors 12, no. 12: 1144. https://0-doi-org.brum.beds.ac.uk/10.3390/bios12121144